Alpha-synuclein and polyunsaturated fatty acids promote clathrin-mediated endocytosis and synaptic vesicle recycling
- PMID: 18980610
- PMCID: PMC2694501
- DOI: 10.1111/j.1600-0854.2008.00853.x
Alpha-synuclein and polyunsaturated fatty acids promote clathrin-mediated endocytosis and synaptic vesicle recycling
Abstract
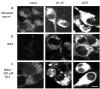
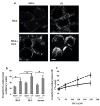
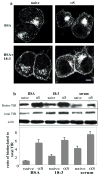
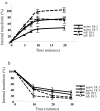
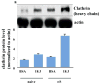
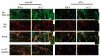
Alpha-synuclein (alphaS) is an abundant neuronal cytoplasmic protein implicated in Parkinson's disease (PD), but its physiological function remains unknown. Consistent with its having structural motifs shared with class A1 apolipoproteins, alphaS can reversibly associate with membranes and help regulate membrane fatty acid composition. We previously observed that variations in alphaS expression level in dopaminergic cultured cells or brains are associated with changes in polyunsaturated fatty acid (PUFA) levels and altered membrane fluidity. We now report that alphaS acts with PUFAs to enhance the internalization of the membrane-binding dye, FM 1-43. Specifically, alphaS expression coupled with exposure to physiological levels of certain PUFAs enhanced clathrin-mediated endocytosis in neuronal and non-neuronal cultured cells. Moreover, alphaS expression and PUFA-enhanced basal and -evoked synaptic vesicle (SV) endocytosis in primary hippocampal cultures of wild type (wt) and genetically depleted alphaS mouse brains. We suggest that alphaS and PUFAs normally function in endocytic mechanisms and are specifically involved in SV recycling upon neuronal stimulation.
Figures
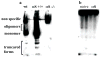





References
-
- Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364(9440):1169–1171. - PubMed
-
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841. - PubMed
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. - PubMed
-
- Choi W, Zibaee S, Jakes R, Serpell LC, Davletov B, Crowther RA, Goedert M. Mutation E46K increases phospholipid binding and assembly into filaments of human alpha-synuclein. FEBS Lett. 2004;576(3):363–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

