Long-term in vitro reactivity for human leukocyte antigen antibodies and comparison of detection using serum versus plasma
- PMID: 18980615
- PMCID: PMC3058293
- DOI: 10.1111/j.1537-2995.2008.01955.x
Long-term in vitro reactivity for human leukocyte antigen antibodies and comparison of detection using serum versus plasma
Abstract
Background: Human leukocyte antigen (HLA) antibodies are a possible cause of transfusion-related acute lung injury (TRALI), and fluorescent bead assays are often used for antibody detection. Serum is the manufacturer's recommended sample, but plasma may be easier to obtain for studies of HLA antibody prevalence and TRALI case investigations.
Study design and methods: Specimens were obtained from 44 multiparous females positive for the presence of HLA antibodies by lymphocytotoxicity testing at least 13 years prior and from 1000 contemporary blood donors. Screening tests were performed using a multiplex bead-based assay. In addition to comparing results obtained with paired plasma and serum samples, the effects of storage at 4 degrees C for 1 week and of multiple freeze-thaw cycles were evaluated.
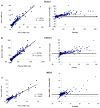
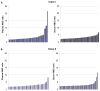
Results: Of 42 evaluable subjects with HLA antibodies documented more than 13 years earlier, only 1 showed loss of detectable antibodies, with 39 (93%) positive in the screening assay for HLA Class I and 24 (57%) positive in the screening assay for HLA Class II antibodies. In 968 evaluable contemporary donors, 291 screened positive for the presence of HLA Class I and 206 for HLA Class II antibodies using a low assay cutoff. Screening test concordance using paired plasma and serum samples was high, particularly for subjects with higher-level antibodies. Refrigeration of samples for 1 week did not significantly affect assay results, while repeated freeze-thaw cycles caused a decrement in signal level.
Conclusion: Serum and plasma samples gave concordant results in the majority of cases, particularly for specimens with higher-level antibodies. High-level HLA antibodies were present in most individuals for more than 13 years.
Figures

References
-
- Holness L, Knippen MA, Simmons L, Lachenbruch PA. Fatalities caused by TRALI. Transfus Med Rev. 2004;18:184–188. - PubMed
-
- Starkey J, Kapler R. Transfusion Recipient Fatalities Reported to the Food and Drug Administration, FY2004–2006. ABC Newsletter; Washington, DC: 2007. p. 21.
-
- Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25:573–577. - PubMed
-
- Cooling L. Transfusion-related acute lung injury. Jama. 2002;288:315–316. author reply 316. - PubMed
-
- Nicolle AL, Chapman CE, Carter V, Wallis JP. Transfusion-related acute lung injury caused by two donors with anti-human leucocyte antigen class II antibodies: a look-back investigation. Transfus Med. 2004;14:225–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01-HB-47172/HB/NHLBI NIH HHS/United States
- N01-HB-47171/HB/NHLBI NIH HHS/United States
- N01 HB057181/HL/NHLBI NIH HHS/United States
- N01-HB-47170/HB/NHLBI NIH HHS/United States
- N01-HB-47175/HB/NHLBI NIH HHS/United States
- N01 HB047168/HL/NHLBI NIH HHS/United States
- N01-HB-47174/HB/NHLBI NIH HHS/United States
- N01-HB-47169/HB/NHLBI NIH HHS/United States
- N01-HB-47173/HB/NHLBI NIH HHS/United States
- HHSN268200517181C/HL/NHLBI NIH HHS/United States
- N01-HB-47168/HB/NHLBI NIH HHS/United States
- N01-HB-57181/HB/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

