Regulation of pancreatic tumor cell proliferation and chemoresistance by the histone methyltransferase enhancer of zeste homologue 2
- PMID: 18980972
- PMCID: PMC2690708
- DOI: 10.1158/1078-0432.CCR-08-1013
Regulation of pancreatic tumor cell proliferation and chemoresistance by the histone methyltransferase enhancer of zeste homologue 2
Abstract
Purpose: Enhancer of zeste homologue 2 (EZH2), a histone methyltransferase, plays a key role in transcriptional repression through chromatin remodeling. Our objectives were to determine the expression pattern of EZH2 and to assess the anticancer effect of EZH2 depletion in pancreatic cancer cells.
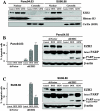
Experimental design: Immunohistochemistry and cytosolic/nuclear fractionation were done to determine the expression pattern of EZH2 in normal pancreas and human pancreatic tumors. We used RNA interference, Western blotting, reverse transcription-PCR, and chromatin immunoprecipitation to study the effect of EZH2 depletion on pancreatic cancer cell proliferation and survival.
Results: We detected nuclear overexpression of EZH2 in pancreatic cancer cell lines and in 71 of 104 (68%) cases of human pancreatic adenocarcinomas. EZH2 nuclear accumulation was more frequent in poorly differentiated pancreatic adenocarcinomas (31 of 34 cases; P<0.001). We found that genetic depletion of EZH2 results in reexpression of p27(Kip1) and decreased pancreatic cancer cell proliferation. Moreover, we showed that EZH2 depletion sensitized pancreatic cancer cells to doxorubicin and gemcitabine, which leads to a significant induction of apoptosis, suggesting that the combination of EZH2 inhibitors and standard chemotherapy could be a superior potential treatment for pancreatic cancer.
Conclusions: Our results show nuclear accumulation of EZH2 as a hallmark of poorly differentiated pancreatic adenocarcinoma; identify the tumor suppressor p27(Kip1) as a new target gene of EZH2; show that EZH2 nuclear overexpression contributes to pancreatic cancer cell proliferation; and suggest EZH2 as a potential therapeutic target for the treatment of pancreatic cancer.
Figures



References
-
- Jaffee EM, Hruban RH, Canto M, Kern SE. Focus on pancreas cancer. Cancer Cell. 2002;2:25–8. - PubMed
-
- Santos-Rosa H, Caldas C. Chromatin modifier enzymes, the histone code and cancer. Eur J Cancer. 2005;41:2381–402. - PubMed
-
- Cao R, Wang L, Wang H, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–43. - PubMed
-
- Viré E, Brenner C, Deplus R, Blanchon L, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;16(439):871–74. - PubMed
-
- Cardoso C, Mignon C, Hetet G, Grandchamps B, Fontes M, Colleaux L. The human EZH2 gene: genomic organisation and revised mapping in 7q35 within the critical region for malignant myeloid disorders. Eur J Hum Genet. 2000;8:174–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

