Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas
- PMID: 18981004
- PMCID: PMC3671765
- DOI: 10.1158/1078-0432.CCR-08-0260
Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas
Abstract
Purpose: Although patients with newly diagnosed WHO grade 3 malignant glioma have a more favorable prognosis than those with WHO grade 4 malignant glioma, salvage therapies following recurrence offer essentially palliative benefit. We did a phase II trial of bevacizumab, a monoclonal antibody to vascular endothelial growth factor, in combination with irinotecan for patients with recurrent grade 3 malignant glioma.
Experimental design: Upon documentation of adequate safety among an initial cohort of nine patients treated with bevacizumab (10 mg/kg) and irinotecan every 14 days, a second cohort (n=24) was treated with bevacizumab (15 mg/kg) every 3 weeks with irinotecan on days 1, 8, 22, and 29 of each 42-day cycle. For both cohorts, the dose of irinotecan was 340 mg/m(2) for patients on enzyme-inducing antiepileptic drugs (EIAED) and 125 mg/m(2) for patients not on EIAEDs. After each 6-week cycle, patients were evaluated with a physical examination and magnetic resonance imaging.
Results: The 6-month progression-free survival was 55% (95% confidence interval, 36-70%). The 6-month overall survival was 79% (95% confidence interval, 61-89%). Twenty patients (61%) had at least a partial response. Outcome did not differ between the two treatment cohorts. Significant adverse events were infrequent and included a central nervous system hemorrhage in one patient, and one patient who developed thrombotic thrombocytopenic purpura.
Conclusion: Bevacizumab and irinotecan is an active regimen with acceptable toxicity for patients with recurrent WHO grade 3 malignant glioma.
Conflict of interest statement
No potential conflicts of interest were disclosed.
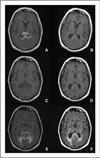
Figures

References
-
- Nieder C, Andratschke N, Wiedenmann N, Busch R, Grosu AL, Molls M. Radiotherapy for high-grade gliomas: does altered fractionation improve the outcome? Strahlenther Onkol. 2004;180:401–407. - PubMed
-
- Fine HA, Dear KB, Loeffler JS, Black PM, Canellos GP. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–2597. - PubMed
-
- Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. - PubMed
-
- Levin VA, Wilson CB, Davis R, Wara WM, Pischer TL, Irwin L. A phase III comparison of BCNU, hydroxyurea, and radiation therapy to BCNU and radiation therapy for treatment of primary malignant gliomas. J Neurosurg. 1979;51:526–532. - PubMed
-
- Buckner JC, Schomberg PJ, McGinnis WL, et al. A phase III study of radiation therapy plus carmustine with or without recombinant interferon-α in the treatment of patients with newly diagnosed high-grade glioma. Cancer. 2001;92:420–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

