Proteome-wide identification of poly(ADP-ribose) binding proteins and poly(ADP-ribose)-associated protein complexes
- PMID: 18981049
- PMCID: PMC2602769
- DOI: 10.1093/nar/gkn771
Proteome-wide identification of poly(ADP-ribose) binding proteins and poly(ADP-ribose)-associated protein complexes
Abstract
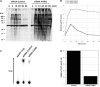
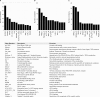
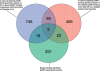
Poly(ADP-ribose) (pADPr) is a polymer assembled from the enzymatic polymerization of the ADP-ribosyl moiety of NAD by poly(ADP-ribose) polymerases (PARPs). The dynamic turnover of pADPr within the cell is essential for a number of cellular processes including progression through the cell cycle, DNA repair and the maintenance of genomic integrity, and apoptosis. In spite of the considerable advances in the knowledge of the physiological conditions modulated by poly(ADP-ribosyl)ation reactions, and notwithstanding the fact that pADPr can play a role of mediator in a wide spectrum of biological processes, few pADPr binding proteins have been identified so far. In this study, refined in silico prediction of pADPr binding proteins and large-scale mass spectrometry-based proteome analysis of pADPr binding proteins were used to establish a comprehensive repertoire of pADPr-associated proteins. Visualization and modeling of these pADPr-associated proteins in networks not only reflect the widespread involvement of poly(ADP-ribosyl)ation in several pathways but also identify protein targets that could shed new light on the regulatory functions of pADPr in normal physiological conditions as well as after exposure to genotoxic stimuli.
Figures




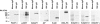

References
-
- Nishizuka Y, Ueda K, Honjo T, Hayaishi O. Enzymic adenosine diphosphate ribosylation of histone and poly adenosine diphosphate ribose synthesis in rat liver nuclei. J. Biol. Chem. 1968;243:3765–3767. - PubMed
-
- Pleschke JM, Kleczkowska HE, Strohm M, Althaus FR. Poly(ADP-ribose) binds to specific domains in DNA damage checkpoint proteins. J. Biol. Chem. 2000;275:40974–40980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

