Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation
- PMID: 18981092
- PMCID: PMC2605669
- DOI: 10.4049/jimmunol.181.10.6747
Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation
Abstract
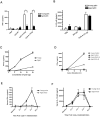
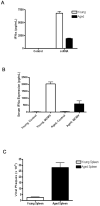
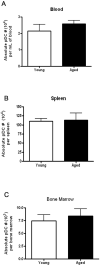
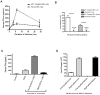
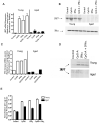
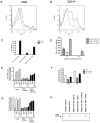
Plasmacytoid dendritic cells (pDCs) are innate sensors that produce IFN-alpha in response to viral infections. Determining how aging alters the cellular and molecular function of these cells may provide an explanation of increased susceptibility of older people to viral infections. Hence, we examined whether aging critically impairs pDC function during infection with HSV-2, a viral pathogen that activates TLR9. We found that impaired IFN-alpha production by aged murine pDCs led to impaired viral clearance with aging. Upon TLR9 activation, aged pDCs displayed defective up-regulation of IFN-regulatory factor 7, a key adaptor in the type I IFN pathway, as compared with younger counterparts. Aged pDCs had more oxidative stress, and reducing oxidative stress in aged pDCs partly recovered the age-induced IFN-alpha defect during TLR9 activation. In sum, aging impairs the type I IFN pathway in pDCs, and this alteration may contribute to the increased susceptibility of older people to certain viral infections.
Figures


Similar articles
-
HIV-1 gp120 impairs the induction of B cell responses by TLR9-activated plasmacytoid dendritic cells.J Immunol. 2012 Dec 1;189(11):5257-65. doi: 10.4049/jimmunol.1201905. Epub 2012 Oct 24. J Immunol. 2012. PMID: 23100517 Free PMC article.
-
Bruton's tyrosine kinase regulates TLR9 but not TLR7 signaling in human plasmacytoid dendritic cells.Eur J Immunol. 2014 Apr;44(4):1130-6. doi: 10.1002/eji.201344030. Epub 2014 Jan 20. Eur J Immunol. 2014. PMID: 24375473
-
Diminished secretion and function of IL-29 is associated with impaired IFN-α response of neonatal plasmacytoid dendritic cells.J Leukoc Biol. 2019 Nov;106(5):1177-1185. doi: 10.1002/JLB.4A0518-189R. Epub 2019 Jun 18. J Leukoc Biol. 2019. PMID: 31211458 Free PMC article.
-
Myxoma virus induces type I interferon production in murine plasmacytoid dendritic cells via a TLR9/MyD88-, IRF5/IRF7-, and IFNAR-dependent pathway.J Virol. 2011 Oct;85(20):10814-25. doi: 10.1128/JVI.00104-11. Epub 2011 Aug 10. J Virol. 2011. PMID: 21835795 Free PMC article.
-
Signalling pathways leading to IFN-alpha production in human plasmacytoid dendritic cell and the possible use of agonists or antagonists of TLR7 and TLR9 in clinical indications.J Intern Med. 2009 Jan;265(1):43-57. doi: 10.1111/j.1365-2796.2008.02050.x. J Intern Med. 2009. PMID: 19093959 Review.
Cited by
-
Type I Interferon Production of Plasmacytoid Dendritic Cells under Control.Int J Mol Sci. 2021 Apr 18;22(8):4190. doi: 10.3390/ijms22084190. Int J Mol Sci. 2021. PMID: 33919546 Free PMC article. Review.
-
Functional genomics of inflamm-aging and immunosenescence.Brief Funct Genomics. 2022 Jan 25;21(1):43-55. doi: 10.1093/bfgp/elab009. Brief Funct Genomics. 2022. PMID: 33690792 Free PMC article. Review.
-
Leukocyte function in the aging immune system.J Leukoc Biol. 2010 Jun;87(6):1001-9. doi: 10.1189/jlb.0809542. Epub 2010 Mar 3. J Leukoc Biol. 2010. PMID: 20200405 Free PMC article. Review.
-
Alphaherpesvirus-induced activation of plasmacytoid dendritic cells depends on the viral glycoprotein gD and is inhibited by non-infectious light particles.PLoS Pathog. 2021 Nov 29;17(11):e1010117. doi: 10.1371/journal.ppat.1010117. eCollection 2021 Nov. PLoS Pathog. 2021. PMID: 34843605 Free PMC article.
-
Sources of Type I Interferons in Infectious Immunity: Plasmacytoid Dendritic Cells Not Always in the Driver's Seat.Front Immunol. 2019 Apr 12;10:778. doi: 10.3389/fimmu.2019.00778. eCollection 2019. Front Immunol. 2019. PMID: 31031767 Free PMC article. Review.
References
-
- Pawelec G, Barnett Y, Forsey R, Frasca D, Globerson A, McLeod J, Caruso C, Franceschi C, Fulop T, Gupta S, Mariani E, Mocchegiani E, Solana R. T cells and aging, January 2002 update. Front Biosci. 2002;7:d1056–1183. - PubMed
-
- Linton P-J, Dorschkind K. Age related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. - PubMed
-
- Yung R. Changes in immune function with age. Rheum. Dis. Clin. N. Am. 2000;26:455–473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

