Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense
- PMID: 18981129
- PMCID: PMC2596683
- DOI: 10.4049/jimmunol.181.10.7090
Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense
Abstract
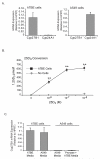
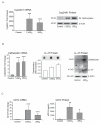
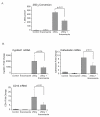
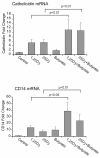
The role of vitamin D in innate immunity is increasingly recognized. Recent work has identified a number of tissues that express the enzyme 1alpha-hydroxylase and are able to activate vitamin D. This locally produced vitamin D is believed to have important immunomodulatory effects. In this paper, we show that primary lung epithelial cells express high baseline levels of activating 1alpha-hydroxylase and low levels of inactivating 24-hydroxylase. The result of this enzyme expression is that airway epithelial cells constitutively convert inactive 25-dihydroxyvitamin D(3) to the active 1,25-dihydroxyvitamin D(3). Active vitamin D that is generated by lung epithelium leads to increased expression of vitamin D-regulated genes with important innate immune functions. These include the cathelicidin antimicrobial peptide gene and the TLR coreceptor CD14. dsRNA increases the expression of 1alpha-hydroxylase, augments the production of active vitamin D, and synergizes with vitamin D to increase expression of cathelicidin. In contrast to induction of the antimicrobial peptide, vitamin D attenuates dsRNA-induced expression of the NF-kappaB-driven gene IL-8. We conclude that primary epithelial cells generate active vitamin D, which then influences the expression of vitamin D-driven genes that play a major role in host defense. Furthermore, the presence of vitamin D alters induction of antimicrobial peptides and inflammatory cytokines in response to viruses. These observations suggest a novel mechanism by which local conversion of inactive to active vitamin D alters immune function in the lung.
Figures
References
-
- Berger U, Wilson P, McClelland RA, Colston K, Haussler MR, Pike JW, Coombes RC. Immunocytochemical detection of 1,25-dihydroxyvitamin D receptors in normal human tissues. J Clin Endocrinol Metab. 1988;67:607–613. - PubMed
-
- FRASER DR, KODICEK E. Unique Biosynthesis by Kidney of a Biologically Active Vitamin D Metabolite. 1970;228:764–766. - PubMed
-
- ZEHNDER D, BLAND R, WALKER EA, BRADWELL AR, HOWIE AJ, HEWISON M, STEWART PM. Expression of 25-Hydroxyvitamin D3-1{alpha}-Hydroxylase in the Human Kidney. J Am Soc Nephrol. 1999;10:2465–2473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

