Toll-like receptor 4 restricts retinal progenitor cell proliferation
- PMID: 18981228
- PMCID: PMC2575781
- DOI: 10.1083/jcb.200804010
Toll-like receptor 4 restricts retinal progenitor cell proliferation
Abstract
Retinal neurogenesis ceases by the early postnatal period, although retinal progenitor cells (RPCs) persist throughout life. In this study, we show that in the mammalian eye, the function of Toll-like receptor 4 (TLR4) extends beyond regulation of the innate immune response; it restricts RPC proliferation. In TLR4-deficient mice, enhanced proliferation of cells reminiscent of RPCs is evident during the early postnatal period. In vitro experiments demonstrate that TLR4 acts as an intrinsic regulator of RPC fate decision. Increased TLR4 expression in the eye correlates with the postnatal cessation of cell proliferation. However, deficient TLR4 expression is not sufficient to extend the proliferative period but rather contributes to resumption of proliferation in combination with growth factors. Proliferation in vivo is inhibited by both MyD88-dependent and -independent pathways, similar to the mechanisms activated by TLR4 in immune cells. Thus, our study attributes a novel role to TLR4 as a negative regulator of RPC proliferation.
Figures
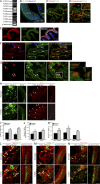
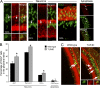
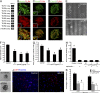

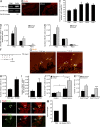
References
-
- Ahmad, I., L. Tang, and H. Pham. 2000. Identification of neural progenitors in the adult mammalian eye. Biochem. Biophys. Res. Commun. 270:517–521. - PubMed
-
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511. - PubMed
-
- Anderson, K.V., L. Bokla, and C. Nusslein-Volhard. 1985. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 42:791–798. - PubMed
-
- Blanks, J.C., and D. Bok. 1977. An autoradiographic analysis of postnatal cell proliferation in the normal and degenerative mouse retina. J. Comp. Neurol. 174:317–327. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

