ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity
- PMID: 18981236
- PMCID: PMC2585848
- DOI: 10.1084/jem.20080840
ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity
Abstract
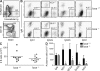
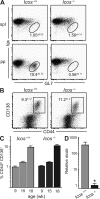
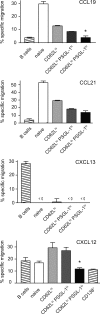
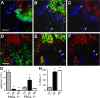
The role of specialized follicular helper T (T(FH)) cells in the germinal center has become well recognized, but it is less clear how effector T cells govern the extrafollicular response, the dominant pathway of high-affinity, isotype-switched autoantibody production in the MRL/MpJ-Fas(lpr) (MRL(lpr)) mouse model of lupus. MRL(lpr) mice lacking the Icos gene have impaired extrafollicular differentiation of immunoglobulin (Ig) G(+) plasma cells accompanied by defects in CXC chemokine receptor (CXCR) 4 expression, interleukin (IL) 21 secretion, and B cell helper function in CD4 T cells. These phenotypes reflect the selective loss of a population of T cells marked by down-regulation of P-selectin glycoprotein ligand 1 (PSGL-1; also known as CD162). PSGL-1(lo) T cells from MRL(lpr) mice express CXCR4, localize to extrafollicular sites, and uniquely mediate IgG production through IL-21 and CD40L. In other autoimmune strains, PSGL-1(lo) T cells are also abundant but may exhibit either a follicular or extrafollicular phenotype. Our findings define an anatomically distinct extrafollicular population of cells that regulates plasma cell differentiation in chronic autoimmunity, indicating that specialized humoral effector T cells akin to T(FH) cells can occur outside the follicle.
Figures




References
-
- Reinhardt, R.L., S.J. Kang, H.E. Liang, and R.M. Locksley. 2006. T helper cell effector fates–who, how and where? Curr. Opin. Immunol. 18:271–277. - PubMed
-
- Mosmann, T.R., and R.L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145–173. - PubMed
-
- Abbas, A.K., K.M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature. 383:787–793. - PubMed
-
- Kaplan, M.H., U. Schindler, S.T. Smiley, and M.J. Grusby. 1996. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 4:313–319. - PubMed
-
- Okada, T., and J.G. Cyster. 2006. B cell migration and interactions in the early phase of antibody responses. Curr. Opin. Immunol. 18:278–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

