Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells
- PMID: 18981239
- PMCID: PMC2585831
- DOI: 10.1084/jem.20081786
Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells
Abstract
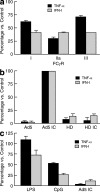
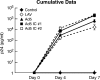
The STEP HIV vaccine trial, which evaluated a replication-defective adenovirus type 5 (Ad5) vector vaccine, was recently stopped. The reasons for this included lack of efficacy of the vaccine and a twofold increase in the incidence of HIV acquisition among vaccinated recipients with increased Ad5-neutralizing antibody titers compared with placebo recipients. To model the events that might be occurring in vivo, the effect on dendritic cells (DCs) of Ad5 vector alone or treated with neutralizing antiserum (Ad5 immune complexes [IC]) was compared. Ad5 IC induced more notable DC maturation, as indicated by increased CD86 expression, decreased endocytosis, and production of tumor necrosis factor and type I interferons. We found that DC stimulation by Ad5 IC was mediated by the Fcgamma receptor IIa and Toll-like receptor 9 interactions. DCs treated with Ad5 IC also induced significantly higher stimulation of Ad5-specific CD8 T cells equipped with cytolytic machinery. In contrast to Ad5 vectors alone, Ad5 IC caused significantly enhanced HIV infection in DC-T cell cocultures. The present results indicate that Ad5 IC activates a DC-T cell axis that, together with the possible persistence of the Ad5 vaccine in seropositive individuals, may set up a permissive environment for HIV-1 infection, which could account for the increased acquisition of HIV-1 infection among Ad5 seropositive vaccine recipients.
Figures



References
-
- Pantaleo, G., and R.A. Koup. 2004. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 10:806–810. - PubMed
-
- Schmitz, J.E., M.J. Kuroda, S. Santra, V.G. Sasseville, M.A. Simon, M.A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B.J. Scallon, et al. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 283:857–860. - PubMed
-
- Betts, M.R., C.M. Gray, J.H. Cox, and G. Ferrari. 2006. Antigen-specific T-cell-mediated immunity after HIV-1 infection: implications for vaccine control of HIV development. Expert Rev. Vaccines. 5:505–516. - PubMed
-
- Pantaleo, G., and A. Harari. 2006. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat. Rev. Immunol. 6:417–423. - PubMed
-
- Johnston, M.I., and A.S. Fauci. 2007. An HIV vaccine—evolving concepts. N. Engl. J. Med. 356:2073–2081. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

