Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes
- PMID: 18981294
- PMCID: PMC2651012
- DOI: 10.1182/blood-2008-02-141937
Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes
Abstract
TNFRSF13B encodes transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), a B cell- specific tumor necrosis factor (TNF) receptor superfamily member. Both biallelic and monoallelic TNFRSF13B mutations were identified in patients with common variable immunodeficiency disorders. The genetic complexity and variable clinical presentation of TACI deficiency prompted us to evaluate the genetic, immunologic, and clinical condition in 50 individuals with TNFRSF13B alterations, following screening of 564 unrelated patients with hypogammaglobulinemia. We identified 13 new sequence variants. The most frequent TNFRSF13B variants (C104R and A181E; n=39; 6.9%) were also present in a heterozygous state in 2% of 675 controls. All patients with biallelic mutations had hypogammaglobulinemia and nearly all showed impaired binding to a proliferation-inducing ligand (APRIL). However, the majority (n=41; 82%) of the pa-tients carried monoallelic changes in TNFRSF13B. Presence of a heterozygous mutation was associated with antibody deficiency (P< .001, relative risk 3.6). Heterozygosity for the most common mutation, C104R, was associated with disease (P< .001, relative risk 4.2). Furthermore, heterozygosity for C104R was associated with low numbers of IgD(-)CD27(+) B cells (P= .019), benign lymphoproliferation (P< .001), and autoimmune complications (P= .001). These associations indicate that C104R heterozygosity increases the risk for common variable immunodeficiency disorders and influences clinical presentation.
Figures

 designates a patient with a double mutation on one allele of TNFRSF13B. Compound heterozygous mutated TACI-deficient patients are labeled as indicated in the figure. CRD indicates cysteine-rich domain; stalk, stalk region; and TM, transmembrane domain.
designates a patient with a double mutation on one allele of TNFRSF13B. Compound heterozygous mutated TACI-deficient patients are labeled as indicated in the figure. CRD indicates cysteine-rich domain; stalk, stalk region; and TM, transmembrane domain.

 , healthy donors (n = 5); and
, healthy donors (n = 5); and  , CVID patients without TNFRSF13B mutation (n = 5). The thick gray line in each graph indicates the background staining of a previously described homozygous S144X cell line. Mean values and SDs are shown at the right side of the scatter diagram of each group. Testing for significance was performed with the Student t test. (B) TACI expression and APRIL binding on EBV-transformed cell lines with homozygous or compound heterozygous TNFRSF13B mutations, stained with a polyclonal antibody directed against the TACI extracellular domain (top panels), stained with a monoclonal antibody recognizing the TACI extracellular domain (middle panels), and stained with a Flag-tagged APRIL construct (bottom panels). Thick black lines indicate patients; thick dashed black lines, healthy donors; and gray filled histograms, patients with TNFRSF13B null mutation (S144X homozygous, negative control).
, CVID patients without TNFRSF13B mutation (n = 5). The thick gray line in each graph indicates the background staining of a previously described homozygous S144X cell line. Mean values and SDs are shown at the right side of the scatter diagram of each group. Testing for significance was performed with the Student t test. (B) TACI expression and APRIL binding on EBV-transformed cell lines with homozygous or compound heterozygous TNFRSF13B mutations, stained with a polyclonal antibody directed against the TACI extracellular domain (top panels), stained with a monoclonal antibody recognizing the TACI extracellular domain (middle panels), and stained with a Flag-tagged APRIL construct (bottom panels). Thick black lines indicate patients; thick dashed black lines, healthy donors; and gray filled histograms, patients with TNFRSF13B null mutation (S144X homozygous, negative control).
 , heterozygous TNFRSF13B mutations other than C104R; ○, C104R heterozygous mutations; and
, heterozygous TNFRSF13B mutations other than C104R; ○, C104R heterozygous mutations; and  , mean values for each patient group. Testing for significant differences between patient groups was performed with the Student t test.
, mean values for each patient group. Testing for significant differences between patient groups was performed with the Student t test.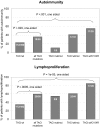
References
-
- Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. - PubMed
-
- Wang J, Cunningham-Rundles C. Treatment and outcome of autoimmune hematologic disease in common variable immunodeficiency (CVID). J Autoimmun. 2005;25:57–62. - PubMed
-
- Wehr C, Kivioja T, Schmitt C, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. - PubMed
-
- Chapel H, Lucas M, Lee M, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

