Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole
- PMID: 18981464
- PMCID: PMC2651094
- DOI: 10.1200/JCO.2008.17.0829
Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole
Abstract
Purpose: To evaluate the prognostic and predictive value of Ki-67 labeling index (LI) in a trial comparing letrozole (Let) with tamoxifen (Tam) as adjuvant therapy in postmenopausal women with early breast cancer.
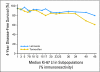
Patients and methods: Breast International Group (BIG) trial 1-98 randomly assigned 8,010 patients to four treatment arms comparing Let and Tam with sequences of each agent. Of 4,922 patients randomly assigned to receive 5 years of monotherapy with either agent, 2,685 had primary tumor material available for central pathology assessment of Ki-67 LI by immunohistochemistry and had tumors confirmed to express estrogen receptors after central review. The prognostic and predictive value of centrally measured Ki-67 LI on disease-free survival (DFS) were assessed among these patients using proportional hazards modeling, with Ki-67 LI values dichotomized at the median value of 11%.
Results: Higher values of Ki-67 LI were associated with adverse prognostic factors and with worse DFS (hazard ratio [HR; high:low] = 1.8; 95% CI, 1.4 to 2.3). The magnitude of the treatment benefit for Let versus Tam was greater among patients with high tumor Ki-67 LI (HR [Let:Tam] = 0.53; 95% CI, 0.39 to 0.72) than among patients with low tumor Ki-67 LI (HR [Let:Tam] = 0.81; 95% CI, 0.57 to 1.15; interaction P = .09).
Conclusion: Ki-67 LI is confirmed as a prognostic factor in this study. High Ki-67 LI levels may identify a patient group that particularly benefits from initial Let adjuvant therapy.
Figures




References
-
- Goldhirsch A, Wood WC, Gelber RD, et al: Progress and promise: Highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 18:1133-1144, 2007 - PubMed
-
- Clahsen PC, Van de Velde CJ, Duval C, et al: The utility of mitotic index, oestrogen receptor and Ki-67 measurements in the creation of novel prognostic indices for node-negative breast cancer. Eur J Surg Oncol 25:356-363, 1999 - PubMed
-
- Gerdes J, Schwab U, Lemke H, et al: Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31:13-20, 1983 - PubMed
-
- Lehr HA, Hansen DA, Kussick S, et al: Assessment of proliferative activity in breast cancer: MIB-1 immunohistochemistry versus mitotic figure count. Hum Pathol 30:1314-1320, 1999 - PubMed
-
- Thor AD, Liu S, Moore DH, et al: Comparison of mitotic index, in vitro bromodeoxyuridine labeling, and MIB-1 assays to quantitate proliferation in breast cancer. J Clin Oncol 17:470-477, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

