Phase I clinical evaluation of rDEN4Delta30-200,201: a live attenuated dengue 4 vaccine candidate designed for decreased hepatotoxicity
- PMID: 18981503
- PMCID: PMC2590927
Phase I clinical evaluation of rDEN4Delta30-200,201: a live attenuated dengue 4 vaccine candidate designed for decreased hepatotoxicity
Abstract
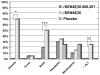
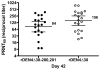
The rDEN4Delta30-200,201 is a live attenuated DENV-4 vaccine candidate specifically designed to further attenuate the rDEN4Delta30 parent virus. In the present study, 28 healthy adult volunteers were randomized to receive either 10(5) plaque-forming unit (PFU) of vaccine (20) or placebo (8) as a single subcutaneous injection. Volunteers were evaluated for safety every other day for 16 days. Serum neutralizing antibody titer against DEN4 was determined at study day 28, 42, and 180. The vaccine infected all vaccinees and was well tolerated without inducing alanine aminotransferase (ALT) elevations. Although virus was not recovered from the serum of any vaccinee, moderate levels of neutralizing antibody were induced in all volunteers. Thus the restricted replication of rDEN4Delta30-200,201 previously documented in animal models was confirmed in humans. The rDEN4Delta30-200,201 is a promising candidate and can be considered for inclusion in a tetravalent dengue virus (DENV) vaccine.
Figures
Similar articles
-
Targeted mutagenesis as a rational approach to dengue virus vaccine development.Curr Top Microbiol Immunol. 2010;338:145-58. doi: 10.1007/978-3-642-02215-9_11. Curr Top Microbiol Immunol. 2010. PMID: 19802584 Free PMC article. Review.
-
rDEN4delta30, a live attenuated dengue virus type 4 vaccine candidate, is safe, immunogenic, and highly infectious in healthy adult volunteers.J Infect Dis. 2005 Mar 1;191(5):710-8. doi: 10.1086/427780. Epub 2005 Jan 27. J Infect Dis. 2005. PMID: 15688284 Clinical Trial.
-
Phase 1 trial of the dengue virus type 4 vaccine candidate rDEN4{Delta}30-4995 in healthy adult volunteers.Am J Trop Med Hyg. 2009 Nov;81(5):834-41. doi: 10.4269/ajtmh.2009.09-0131. Am J Trop Med Hyg. 2009. PMID: 19861619 Free PMC article. Clinical Trial.
-
Evaluation of the Langat/dengue 4 chimeric virus as a live attenuated tick-borne encephalitis vaccine for safety and immunogenicity in healthy adult volunteers.Vaccine. 2008 Feb 13;26(7):882-90. doi: 10.1016/j.vaccine.2007.12.015. Epub 2008 Jan 4. Vaccine. 2008. PMID: 18207289 Free PMC article. Clinical Trial.
-
A recombinant, chimeric tetravalent dengue vaccine candidate based on a dengue virus serotype 2 backbone.Expert Rev Vaccines. 2016;15(4):497-508. doi: 10.1586/14760584.2016.1128328. Epub 2016 Feb 22. Expert Rev Vaccines. 2016. PMID: 26635182 Review.
Cited by
-
Issues related to recent dengue vaccine development.Trop Med Health. 2011 Dec;39(4 Suppl):63-71. doi: 10.2149/tmh.2011-S01. Epub 2011 Aug 6. Trop Med Health. 2011. PMID: 22500138 Free PMC article.
-
Historical discourse on the development of the live attenuated tetravalent dengue vaccine candidate TV003/TV005.Curr Opin Virol. 2020 Aug;43:79-87. doi: 10.1016/j.coviro.2020.09.005. Epub 2020 Oct 23. Curr Opin Virol. 2020. PMID: 33164790 Free PMC article. Review.
-
Neutropenia as an Adverse Event following Vaccination: Results from Randomized Clinical Trials in Healthy Adults and Systematic Review.PLoS One. 2016 Aug 4;11(8):e0157385. doi: 10.1371/journal.pone.0157385. eCollection 2016. PLoS One. 2016. PMID: 27490698 Free PMC article.
-
Understanding the dengue viruses and progress towards their control.Biomed Res Int. 2013;2013:690835. doi: 10.1155/2013/690835. Epub 2013 Jul 9. Biomed Res Int. 2013. PMID: 23936833 Free PMC article. Review.
-
Targeted mutagenesis as a rational approach to dengue virus vaccine development.Curr Top Microbiol Immunol. 2010;338:145-58. doi: 10.1007/978-3-642-02215-9_11. Curr Top Microbiol Immunol. 2010. PMID: 19802584 Free PMC article. Review.
References
-
- Suaya JA, Shepard DS, Beatty ME. Scientific Working Group on Dengue—2006 Report. Geneva: WHO; 2006. Dengue: Burden of Disease and Costs of Illness; pp. 35–49.
-
- World Health Organization. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. Geneva: WHO; 1997.
-
- Sabin A. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. - PubMed
-
- Okuno Y, Fukunaga T, Tadano M, Fukai K, Ikeda T, Sekii K, Ariyoshi H. Serological studies on volunteers inoculated experimentally with a dengue virus strain in 1943. Biken J. 1983;26:161–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
