A magnetic resonance imaging study of intestinal dilation in Trypanosoma cruzi-infected mice deficient in nitric oxide synthase
- PMID: 18981519
- PMCID: PMC2701742
A magnetic resonance imaging study of intestinal dilation in Trypanosoma cruzi-infected mice deficient in nitric oxide synthase
Abstract
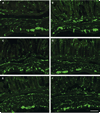
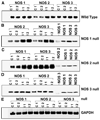
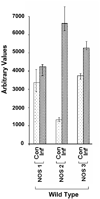
Infection with Trypanosoma cruzi causes megasyndromes of the gastrointestinal (GI) tract. We used magnetic resonance imaging (MRI) to monitor alterations in the GI tract of T. cruzi-infected mice, and to assess the role of nitric oxide (NO) in the development of intestinal dilation. Brazil strain-infected C57BL/6 wild-type (WT) mice exhibited dilatation of the intestines by 30 days post-infection. Average intestine lumen diameter increased by 72%. Levels of intestinal NO synthase (NOS) isoforms, NOS2 and NOS3, were elevated in infected WT mice. Inflammation and ganglionitis were observed in all infected mice. Intestinal dilation was observed in infected WT, NOS1, NOS2, and NOS3 null mice. This study demonstrates that MRI is a useful tool to monitor intestinal dilation in living mice and that these alterations may begin during acute infection. Furthermore, our data strongly suggests that NO may not be the sole contributor to intestinal dysfunction resulting from this infection.
Figures




References
-
- Tait P, Allison D. Imaging of the gastrointestinal tract. Drugs Today (Barc) 2001;37:533–557. - PubMed
-
- Lomas DJ. Technical developments in bowel MRI. Eur Radiol. 2003;13:1058–1071. - PubMed
-
- Lawler LP, Fishman EK. Bladder imaging using multidetector row computed tomography, volume rendering, and magnetic resonance imaging. J Comput Assist Tomogr. 2003;27:553–563. - PubMed
-
- Bruel JM, Gallix B. Multidetector CT and MRI in diseases of the GI tract. J Radiol. 2003;84:499–513. - PubMed
-
- Boczko J, Tar M, Melman A, Jelicks LA, Wittner M, Factor SM, Zhao D, Hafron J, Weiss LM, Tanowitz HB, Christ GJ. Trypanosoma cruzi infection induced changes in the innervation, structure and function of the murine bladder. J Urol. 2005;173:1784–1788. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
