Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury
- PMID: 18981722
- PMCID: PMC2745149
- DOI: 10.4161/cbt.8.1.7092
Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury
Abstract
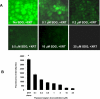
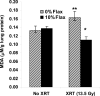
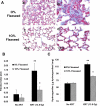
Flaxseed (FS) has high contents of omega-3 fatty acids and lignans with antioxidant properties. Its use in preventing thoracic X-ray radiation therapy (XRT)-induced pneumonopathy has never been evaluated. We evaluated FS supplementation given to mice given before and post-XRT. FS-derived lignans, known for their direct antioxidant properties, were evaluated in abrogating ROS generation in cultured endothelial cells following gamma radiation exposure. Mice were fed 10% FS or isocaloric control diet for three weeks and given 13.5 Gy thoracic XRT. Lungs were evaluated at 24 hours for markers of radiation-induced injury, three weeks for acute lung damage (lipid peroxidation, lung edema and inflammation), and at four months for late lung damage (inflammation and fibrosis). FS-Lignans blunted ROS generation in vitro, resulting from radiation in a dose-dependent manner. FS-fed mice had reduced expression of lung injury biomarkers (Bax, p21 and TGF-beta1) at 24 hours following XRT and reduced oxidative lung damage as measured by malondialdehyde (MDA) levels at 3 weeks following XRT. In addition, FS-fed mice had decreased lung fibrosis as determined by hydroxyproline content and decreased inflammatory cell influx into lungs at 4 months post XRT. Importantly, when Lewis lung carcinoma cells were injected systemically in mice, FS dietary supplementation did not appear to protect lung tumors from responding to thoracic XRT. Dietary FS is protective against pulmonary fibrosis, inflammation and oxidative lung damage in a murine model. Moreover, in this model, tumor radioprotection was not observed. FS lignans exhibited potent radiation-induced ROS scavenging action. Taken together, these data suggest that dietary flaxseed may be clinically useful as an agent to increase the therapeutic index of thoracic XRT by increasing the radiation tolerance of lung tissues.
Figures


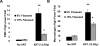
Similar articles
-
Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice.BMC Cancer. 2011 Jun 24;11:269. doi: 10.1186/1471-2407-11-269. BMC Cancer. 2011. PMID: 21702963 Free PMC article.
-
Radiation mitigating properties of the lignan component in flaxseed.BMC Cancer. 2013 Apr 4;13:179. doi: 10.1186/1471-2407-13-179. BMC Cancer. 2013. PMID: 23557217 Free PMC article.
-
Dietary flaxseed modulates the miRNA profile in irradiated and non-irradiated murine lungs: a novel mechanism of tissue radioprotection by flaxseed.Cancer Biol Ther. 2014 Jul;15(7):930-7. doi: 10.4161/cbt.28905. Epub 2014 Apr 22. Cancer Biol Ther. 2014. PMID: 24755684 Free PMC article.
-
Plant extracts and plant-derived compounds: promising players in a countermeasure strategy against radiological exposure.Asian Pac J Cancer Prev. 2014;15(6):2405-25. doi: 10.7314/apjcp.2014.15.6.2405. Asian Pac J Cancer Prev. 2014. PMID: 24761841 Review.
-
Acute Radiation-induced Lung Injury in the Non-human Primate: A Review and Comparison of Mortality and Co-morbidities Using Models of Partial-body Irradiation with Marginal Bone Marrow Sparing and Whole Thorax Lung Irradiation.Health Phys. 2020 Nov;119(5):559-587. doi: 10.1097/HP.0000000000001346. Health Phys. 2020. PMID: 33009295 Free PMC article. Review.
Cited by
-
The Utilization of Plant-Material-Loaded Vesicular Drug Delivery Systems in the Management of Pulmonary Diseases.Curr Issues Mol Biol. 2023 Dec 12;45(12):9985-10017. doi: 10.3390/cimb45120624. Curr Issues Mol Biol. 2023. PMID: 38132470 Free PMC article. Review.
-
Tolfenamic acid decreases c-Met expression through Sp proteins degradation and inhibits lung cancer cells growth and tumor formation in orthotopic mice.Invest New Drugs. 2011 Feb;29(1):41-51. doi: 10.1007/s10637-009-9331-8. Epub 2009 Oct 23. Invest New Drugs. 2011. PMID: 19851711
-
Dietary Flaxseed in Non-Small Cell Lung Cancer Patients Receiving Chemoradiation.J Pulm Respir Med. 2013 Aug 30;3(4):154. doi: 10.4172/2161-105X.1000154. J Pulm Respir Med. 2013. PMID: 24575360 Free PMC article.
-
The Flaxseed-Derived Lignan Phenolic Secoisolariciresinol Diglucoside (SDG) Protects Non-Malignant Lung Cells from Radiation Damage.Int J Mol Sci. 2015 Dec 22;17(1):7. doi: 10.3390/ijms17010007. Int J Mol Sci. 2015. PMID: 26703588 Free PMC article.
-
Reactive Oxygen Species Drive Epigenetic Changes in Radiation-Induced Fibrosis.Oxid Med Cell Longev. 2019 Feb 6;2019:4278658. doi: 10.1155/2019/4278658. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 30881591 Free PMC article. Review.
References
-
- Machtay M. Pulmonary complications of anticancer treatment. 3rd. ed. Churchill Livingston; 2003.
-
- Wang JY, Chen KY, Wang JT, et al. Outcome and prognostic factors for patients with non-small-cell lung cancer and severe radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2002;54:735–741. - PubMed
-
- Robnett TJ, Machtay M, Vines EF, et al. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2000;48:89–94. - PubMed
-
- Hughes-Davies L, Tarbell NJ, Coleman CN, et al. Stage IA-IIB Hodgkin's disease: management and outcome of extensive thoracic involvement. Int J Radiat Oncol Biol Phys. 1997;39:361–369. - PubMed
-
- Marks LB, Fan M, Clough R, et al. Radiation-induced pulmonary injury: symptomatic versus subclinical endpoints. Int J Radiat Biol. 2000;76:469–475. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
