Five-year outcomes of initial patients treated in Botswana's National Antiretroviral Treatment Program
- PMID: 18981769
- PMCID: PMC2853026
- DOI: 10.1097/QAD.0b013e3283129db0
Five-year outcomes of initial patients treated in Botswana's National Antiretroviral Treatment Program
Abstract
Background: Antiretroviral treatment (ART) initiatives have now been established in many sub-Saharan African countries showing early benefits. To date, few results are available concerning long-term clinical outcomes in these treatment programs.
Methods: Response to ART is described in the first HIV-1C-infected adults enrolled in the Botswana Antiretroviral Treatment Program in 2002. Data analysis was conducted on available longitudinal data up to 1st April 2007.
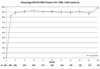
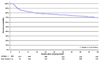
Results: Six hundred thirty-three severely immunodeficient patients with a median CD4+ cell count of 67 cells/microl were initiated on non-nucleoside reverse transcriptase inhibitor-based combination ART and followed for a median of 41.9 months. The median CD4+ cell count increases were 169, 302, and 337 cells/microl at 1, 3, and 5 years, respectively. The percentages of patients with a viral load of less than 400 copies/ml at 1, 3, and 5 years were 91.3, 90.1, and 98.3%, respectively. Seventy-five percent of patients did not miss a single, or missed only one, monthly ART pickup per year with a mean pickup rate of 92.5%. The Kaplan-Meier survival estimates [95% confidence interval (CI)] at 1, 3, and 5 years were 82.7% (81.2 and 84.3%), 79.3% (77.6 and 81.0%), and 79.0% (77.3 and 80.7%), respectively. At 6 months, the risk of treatment modification for anemia was 6.94% (5.9 and 8.0%) for cutaneous hypersensitivity reactions, 1.3% (0.8 and 1.7%), and 1.1% (0.7 and 1.6%) for hepatotoxicity.
Conclusion: This initial group of adults on ART in Botswana had excellent sustained immunologic, virologic, and clinical outcomes for up to 5 years of follow-up with low mortality among those surviving into the second year of ART.
Figures
References
-
- Puvimanasinghe J. MASA Report. Gaborone: Ministry of Health, Botswana; 2007.
-
- Stringer JS, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. Jama. 2006;296(7):782–793. - PubMed
-
- Wester CW, et al. Initial response to highly active antiretroviral therapy in HIV-1C-infected adults in a public sector treatment program in Botswana. J Acquir Immune Defic Syndr. 2005;40(3):336–343. - PubMed
-
- Coetzee D, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. Aids. 2004;18(6):887–895. - PubMed
-
- Weidle PJ, et al. Assessment of a pilot antiretroviral drug therapy programme in Uganda: patients' response, survival, and drug resistance. Lancet. 2002;360(9326):34–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous