Antiretroviral drugs for preventing mother-to-child transmission of HIV in sub-Saharan Africa: balancing efficacy and infant toxicity
- PMID: 18981776
- PMCID: PMC2881583
- DOI: 10.1097/QAD.0b013e3283189bd7
Antiretroviral drugs for preventing mother-to-child transmission of HIV in sub-Saharan Africa: balancing efficacy and infant toxicity
Abstract
Objective: Antiretroviral drugs can prevent mother-to-child transmission of HIV infection, but in-utero antiretroviral exposure may be associated with neurologic symptoms due to mitochondrial toxicity. We sought to identify the currently recommended regimen to prevent mother-to-child transmission that optimally balances risks of pediatric HIV infection and neurologic mitochondrial toxicity.
Design: Published MTCT and mitochondrial toxicity data were used in a decision analytic model of MTCT among women in sub-Saharan Africa.
Methods: We investigated the HIV and mitochondrial toxicity risks associated with no antiretroviral prophylaxis and five recommended regimens ranging from single-dose nevirapine to three-drug antiretroviral therapy (ART). Sensitivity analyses varied all parameters, including infant feeding strategy and the disability of mitochondrial toxicity relative to HIV.
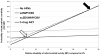
Results: Provision of no antiretroviral drugs is the least effective and least toxic strategy, with 18-month HIV risk of 30.4% and mitochondrial toxicity risk of 0.2% (breastfed infants). With increasing drug number and duration, HIV risk decreases markedly (to 4.9% with three-drug ART), but mitochondrial toxicity risk also increases (to 2.2%, also with three-drug ART). Despite increased toxicity, three-drug ART minimizes total adverse pediatric outcomes (HIV plus mitochondrial toxicity), unless the highest published risks are true for both HIV and mitochondrial toxicity, or the disability from mitochondrial toxicity exceeds 6.4 times that of HIV infection.
Conclusion: The risk of pediatric mitochondrial toxicity from effective regimens to prevent mother-to-child transmission is at least an order of magnitude lower than the risk of HIV infection associated with less-effective regimens. Concern regarding mitochondrial toxicity should not currently limit the use of three-drug ART to prevent mother-to-child transmission where it is available.
Figures

References
-
- Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O'Sullivan MJ, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. - PubMed
-
- Do minguez K, Bertolli J, Fowler M, Peters V, Ortiz I, Melville S, et al. Lack of definitive severe mitochondrial signs and symptoms among deceased HIV-uninfected and HIV-indeterminate children < or = 5 years of age, Pediatric Spectrum of HIV Disease project (PSD), USA. Ann N Y Acad Sci. 2000;918:236–246. - PubMed
-
- Leroy V, Karon JM, Alioum A, Ekpini ER, Meda N, Greenberg AE, et al. Twenty-four month efficacy of a maternal short-course zidovudine regimen to prevent mother-to-child transmission of HIV-1 in West Africa. AIDS. 2002;16:631–641. - PubMed
-
- Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–494. - PubMed
-
- Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind placebo-controlled trial. Lancet. 2002;359:1178–1186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

