Dystroglycan, Tks5 and Src mediated assembly of podosomes in myoblasts
- PMID: 18982058
- PMCID: PMC2572840
- DOI: 10.1371/journal.pone.0003638
Dystroglycan, Tks5 and Src mediated assembly of podosomes in myoblasts
Abstract
Background: Dystroglycan is a ubiquitously expressed cell adhesion receptor best understood in its role as part of the dystrophin glycoprotein complex of mature skeletal muscle. Less is known of the role of dystroglycan in more fundamental aspects of cell adhesion in other cell types, nor of its role in myoblast cell adhesion.
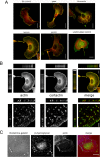
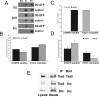
Principal findings: We have examined the role of dystroglycan in the early stages of myoblast adhesion and spreading and found that dystroglycan initially associates with other adhesion proteins in large puncta morphologically similar to podosomes. Using a human SH3 domain phage display library we identified Tks5, a key regulator of podosomes, as interacting with beta-dystroglycan. We verified the interaction by immunoprecipitation, GST-pulldown and immunfluorescence localisation. Both proteins localise to puncta during early phases of spreading, but importantly following stimulation with phorbol ester, also localise to structures indistinguishable from podosomes. Dystroglycan overexpression inhibited podosome formation by sequestering Tks5 and Src. Mutation of dystroglycan tyrosine 890, previously identified as a Src substrate, restored podosome formation.
Conclusions: We propose therefore, that Src-dependent phosphorylation of beta-dystroglycan results in the formation of a Src/dystroglycan complex that drives the SH3-mediated association between dystroglycan and Tks5 which together regulate podosome formation in myoblasts.
Conflict of interest statement
Figures







References
-
- Anderson C, Winder SJ, Borycki A-G. Dystroglycan protein distribution coincides with basement membranes and muscle differentiation during mouse embryogenesis. Dev Dyn. 2007;236:2627–2635. - PubMed
-
- Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends in Cell Biology. 2007;17:107–117. - PubMed
-
- Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–157. - PubMed
-
- Zhang D, Udagawa N, Nakamura I, Murakami H, Saito S, et al. The small GTP-binding protein, rho p21, is involved in bone resorption by regulating cytoskeletal organization in osteoclasts. J Cell Sci. 1995;108:2285–2292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

