Molecular determinants of a symbiotic chronic infection
- PMID: 18983260
- PMCID: PMC2770587
- DOI: 10.1146/annurev.genet.42.110807.091427
Molecular determinants of a symbiotic chronic infection
Abstract
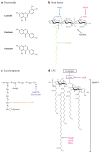
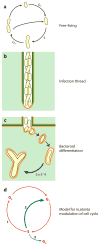
Rhizobial bacteria colonize legume roots for the purpose of biological nitrogen fixation. A complex series of events, coordinated by host and bacterial signal molecules, underlie the development of this symbiotic interaction. Rhizobia elicit de novo formation of a novel root organ within which they establish a chronic intracellular infection. Legumes permit rhizobia to invade these root tissues while exerting control over the infection process. Once rhizobia gain intracellular access to their host, legumes also strongly influence the process of bacterial differentiation that is required for nitrogen fixation. Even so, symbiotic rhizobia play an active role in promoting their goal of host invasion and chronic persistence by producing a variety of signal molecules that elicit changes in host gene expression. In particular, rhizobia appear to advocate for their access to the host by producing a variety of signal molecules capable of suppressing a general pathogen defense response.
Figures


References
-
- Albus U, Baier R, Holst O, Puhler A, Niehaus K. Suppression of an elicitor-induced oxidative burst in Medicago sativa cell-cultures by Sinorhizobium meliloti lipopolysaccharides. New Phytol. 2001;151:597–606. - PubMed
-
- Allaway D, Lodwig EM, Crompton LA, Wood M, Parsons R, et al. Identification of alanine dehydrogenase and its role in mixed secretion of ammonium and alanine by pea bacteroids. Mol Microbiol. 2000;36:508–15. - PubMed
-
- Alunni B, Kevei Z, Redondo-Nieto M, Kondorosi A, Mergaert P, Kondorosi E. Genomic organization and evolutionary insights on GRP and NCR genes, two large nodule-specific gene families in Medicago truncatula. Mol Plant Microbe Interact. 2007;20:1138–48. - PubMed
-
- Ardissone S, Frendo P, Laurenti E, Jantschko W, Obinger C, et al. Purification and physical-chemical characterization of the three hydroperoxidases from the symbiotic bacterium Sinorhizobium meliloti. Biochemistry. 2004;43:12692–99. - PubMed
-
- Ardourel M, Demont N, Debelle F, Maillet F, de Billy F, et al. Rhizobium meliloti lipooligosaccharide nodulation factors: different structural requirements for bacterial entry into target root hair cells and induction of plant symbiotic developmental responses. Plant Cell. 1994;6:1357–74. The structural requirements for S. meliloti NF are more stringent for IT growth than earlier root hair responses. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

