Decoding epithelial signals: critical role for the epidermal growth factor receptor in controlling intestinal transport function
- PMID: 18983445
- PMCID: PMC2630365
- DOI: 10.1111/j.1748-1716.2008.01929.x
Decoding epithelial signals: critical role for the epidermal growth factor receptor in controlling intestinal transport function
Abstract
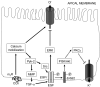
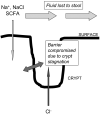
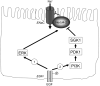
The intestinal epithelium engages in bidirectional transport of fluid and electrolytes to subserve the physiological processes of nutrient digestion and absorption, as well as the elimination of wastes, without excessive losses of bodily fluids that would lead to dehydration. The overall processes of intestinal ion transport, which in turn drive the secretion or absorption of water, are accordingly carefully regulated. We and others have identified the epidermal growth factor receptor (EGFr) as a critical regulator of mammalian intestinal ion transport. In this article, we focus on our studies that have uncovered the intricate signalling mechanisms downstream of EGFr that regulate both chloride secretion and sodium absorption by colonocytes. Emphasis will be placed on the EGFr-associated regulatory pathways that dictate the precise outcome to receptor activation in response to signals that may seem, on their face, to be quite similar if not identical. The concepts to be discussed underlie the ability of the intestinal epithelium to utilize a limited set of signalling effectors to produce a variety of outcomes suitable for varying physiological and pathophysiological demands. Our findings therefore are relevant not only to basic biological principles, but also may ultimately point to new therapeutic targets in intestinal diseases where ion transport is abnormal.
Figures




Similar articles
-
New ways of thinking about (and teaching about) intestinal epithelial function.Adv Physiol Educ. 2008 Mar;32(1):25-34. doi: 10.1152/advan.00092.2007. Adv Physiol Educ. 2008. PMID: 18334565 Review.
-
Epithelial transport in digestive diseases: mice, monolayers, and mechanisms.Am J Physiol Cell Physiol. 2020 Jun 1;318(6):C1136-C1143. doi: 10.1152/ajpcell.00015.2020. Epub 2020 Apr 15. Am J Physiol Cell Physiol. 2020. PMID: 32293934 Free PMC article. Review.
-
Interferon-γ alters downstream signaling originating from epidermal growth factor receptor in intestinal epithelial cells: functional consequences for ion transport.J Biol Chem. 2012 Jan 13;287(3):2144-55. doi: 10.1074/jbc.M111.318139. Epub 2011 Nov 8. J Biol Chem. 2012. PMID: 22069319 Free PMC article.
-
Epidermal growth factor-mediated proliferation and sodium transport in normal and PKD epithelial cells.Biochim Biophys Acta. 2011 Oct;1812(10):1301-13. doi: 10.1016/j.bbadis.2010.10.004. Epub 2010 Oct 16. Biochim Biophys Acta. 2011. PMID: 20959142 Free PMC article. Review.
-
Ion channel TRPV1-dependent activation of PTP1B suppresses EGFR-associated intestinal tumorigenesis.J Clin Invest. 2014 Sep;124(9):3793-806. doi: 10.1172/JCI72340. Epub 2014 Aug 1. J Clin Invest. 2014. PMID: 25083990 Free PMC article.
Cited by
-
The characterization, management, and future considerations for ErbB-family TKI-associated diarrhea.Breast Cancer Res Treat. 2019 May;175(1):5-15. doi: 10.1007/s10549-018-05102-x. Epub 2019 Jan 22. Breast Cancer Res Treat. 2019. PMID: 30671765 Free PMC article. Review.
-
Research Progress on Mechanism and Management of Adverse Drug Reactions of Anlotinib.Drug Des Devel Ther. 2023 Nov 15;17:3429-3437. doi: 10.2147/DDDT.S426898. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 38024530 Free PMC article. Review.
-
Pathophysiology of IBD associated diarrhea.Tissue Barriers. 2018;6(2):e1463897. doi: 10.1080/21688370.2018.1463897. Epub 2018 May 8. Tissue Barriers. 2018. PMID: 29737913 Free PMC article. Review.
-
Development of the rat model of lapatinib-induced diarrhoea.Scientifica (Cairo). 2014;2014:194185. doi: 10.1155/2014/194185. Epub 2014 Jul 7. Scientifica (Cairo). 2014. PMID: 25126444 Free PMC article. Review.
-
Development of a rat model of oral small molecule receptor tyrosine kinase inhibitor-induced diarrhea.Cancer Biol Ther. 2012 Nov;13(13):1269-75. doi: 10.4161/cbt.21783. Epub 2012 Aug 16. Cancer Biol Ther. 2012. PMID: 22895076 Free PMC article.
References
-
- Asfaha S, Bell CJ, Wallace JL, MacNaughton WK. Prolonged colonic epithelial hyporesponsiveness after colitis: role of inducible nitric oxide synthase. American Journal of Physiology - Gastrointestinal and Liver Physiology. 1999;276:G703–G710. - PubMed
-
- Bair CH, Huang JD. Effect of theophylline on the intestinal clearance of drugs in rats. Journal of Pharmacy and Pharmacology. 1992;44:483–486. - PubMed
-
- Barrett KE, Keely SJ. Integrative physiology and pathophysiology of intestinal electrolyte transport. In: Johnson LR, et al., editors. Physiology of the Gastrointestinal Tract. 4 edn Academic Press; San Diego: 2006. pp. 1931–1951.
-
- Bertelsen LS, Barrett KE, Keely SJ. Gs protein-coupled receptor agonists induce transactivation of the epidermal growth factor receptor in T84 cells: implications for epithelial secretory responses. J.Biol.Chem. 2004;279(no. 8):6271–6279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

