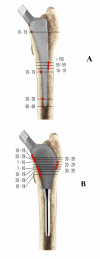
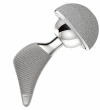
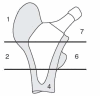
A new short uncemented, proximally fixed anatomic femoral implant with a prominent lateral flare: design rationals and study design of an international clinical trial
- PMID: 18983669
- PMCID: PMC2584636
- DOI: 10.1186/1471-2474-9-147
A new short uncemented, proximally fixed anatomic femoral implant with a prominent lateral flare: design rationals and study design of an international clinical trial
Abstract
Background: Anatomic short femoral prostheses with a prominent lateral flare have the potential to reduce stress-shielding in the femur through a more physiological stress distribution to the proximal femur. We present the design rationale of a new short uncemented, proximally fixed anatomic femoral implant and the study design of a prospective multi-centre trial to collect long-term patient outcome and radiographic follow up data.
Methods: A prospective surveillance study (trial registry NCT00208555) in four European centres (UK, Italy, Spain and Germany) with a follow up period of 15 years will be executed. The recruitment target is 200 subjects, patients between the ages of 18 and 70 admitted for primary cementless unilateral THA will be included. The primary objective is to evaluate the five-year survivorship of the new cementless short stem. The secondary objectives of this investigation are to evaluate the long term survivorship and the clinical performance of the implant, the impact on the subjects health related Quality of Life and the affect of the prosthesis on bone mineral density. Peri- and postoperative complications will be registered. Clinical and radiographic evaluation of prosthesis positioning will be done post-operatively and at 3, 6, 12, 24, 60, 120 and 180 months follow up.
Discussion: Shortening of the distal stem can maximise bone and soft tissue conservation. New stem types have been designed to improve the limitations of traditional implants in primary THA. A new, uncemented femoral short stem is introduced in this paper. A long-term follow up study has been designed to verify stable fixation and to research into the clinical outcome. The results of this trial will be presented as soon as they become available.
Figures
Similar articles
-
Increased risk of periprosthetic femur fractures associated with a unique cementless stem design.Clin Orthop Relat Res. 2015 Jun;473(6):2045-53. doi: 10.1007/s11999-014-4077-9. Epub 2014 Dec 12. Clin Orthop Relat Res. 2015. PMID: 25502478 Free PMC article.
-
Unstable versus stable uncemented femoral stems: a radiological study of periprosthetic bone changes in two types of uncemented stems with different concepts of fixation.Arch Orthop Trauma Surg. 2004 Jul;124(6):382-92. doi: 10.1007/s00402-004-0666-5. Epub 2004 Apr 27. Arch Orthop Trauma Surg. 2004. PMID: 15112084
-
Titanium-titanium modular neck for primary THA. Result of a prospective series of 170 cemented THA with a minimum follow-up of 5 years.Orthop Traumatol Surg Res. 2015 Apr;101(2):137-42. doi: 10.1016/j.otsr.2014.12.013. Epub 2015 Feb 16. Orthop Traumatol Surg Res. 2015. PMID: 25698098 Clinical Trial.
-
Failure of cementless fixation of the femoral component in total hip arthroplasty.Orthop Clin North Am. 1992 Apr;23(2):335-46. Orthop Clin North Am. 1992. PMID: 1570145 Review.
-
[Anchoring principles in hip endoprostheses. I: Prosthesis stem].Unfallchirurg. 2000 Nov;103(11):918-31. doi: 10.1007/s001130050647. Unfallchirurg. 2000. PMID: 11142879 Review. German.
Cited by
-
CORR Insights®: Long-term results and bone remodeling after THA with a short, metaphyseal-fitting anatomic cementless stem.Clin Orthop Relat Res. 2014 Mar;472(3):951-2. doi: 10.1007/s11999-013-3402-z. Epub 2013 Nov 27. Clin Orthop Relat Res. 2014. PMID: 24281992 Free PMC article. No abstract available.
-
Ultra-Short Bone Conserving Cementless Femoral Stem.Hip Pelvis. 2021 Dec;33(4):181-189. doi: 10.5371/hp.2021.33.4.181. Epub 2021 Dec 1. Hip Pelvis. 2021. PMID: 34938687 Free PMC article. Review.
-
The loading patterns of a short femoral stem in total hip arthroplasty: gait analysis at increasing walking speeds and inclines.J Orthop Traumatol. 2018 Aug 17;19(1):14. doi: 10.1186/s10195-018-0504-0. J Orthop Traumatol. 2018. PMID: 30120638 Free PMC article. Clinical Trial.
-
Are short-stem prostheses superior to conventional stem prostheses in primary total hip arthroplasty? A systematic review and meta-analysis of randomised controlled trials.BMJ Open. 2018 Sep 21;8(9):e021649. doi: 10.1136/bmjopen-2018-021649. BMJ Open. 2018. PMID: 30244208 Free PMC article.
-
Clinical outcome of design modifications to the CLS Spotorno Stem in total hip replacement.Joints. 2016 Sep 21;4(3):134-141. doi: 10.11138/jts/2016.4.3.134. eCollection 2016 Jul-Sep. Joints. 2016. PMID: 27900304 Free PMC article.
References
-
- Learmonth ID, Grobler GP, Dall DM, Jandera V. Loss of bone stock with cementless hip arthroplasty. J Arthroplasty. 1995;10:257–263. - PubMed
-
- Kawamura H, Dunbar MJ, Murray P, Bourne RB, Rorabeck CH. The porous coated anatomic total hip replacement. A ten to fourteen-year follow-up study of a cementless total hip arthroplasty. J Bone Joint Surg Am. 2001;83-A:1333–1338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical