Targeting histone deacetylase activity in rheumatoid arthritis and asthma as prototypes of inflammatory disease: should we keep our HATs on?
- PMID: 18983693
- PMCID: PMC2592777
- DOI: 10.1186/ar2489
Targeting histone deacetylase activity in rheumatoid arthritis and asthma as prototypes of inflammatory disease: should we keep our HATs on?
Abstract
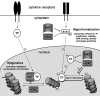
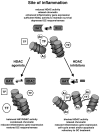
Cellular activation, proliferation and survival in chronic inflammatory diseases is regulated not only by engagement of signal trans-duction pathways that modulate transcription factors required for these processes, but also by epigenetic regulation of transcription factor access to gene promoter regions. Histone acetyl transferases coordinate the recruitment and activation of transcription factors with conformational changes in histones that allow gene promoter exposure. Histone deacetylases (HDACs) counteract histone acetyl transferase activity through the targeting of both histones as well as nonhistone signal transduction proteins important in inflammation. Numerous studies have indicated that depressed HDAC activity in patients with inflammatory airway diseases may contribute to local proinflammatory cytokine production and diminish patient responses to corticosteroid treatment. Recent observations that HDAC activity is depressed in rheumatoid arthritis patient synovial tissue have predicted that strategies restoring HDAC function may be therapeutic in this disease as well. Pharmacological inhibitors of HDAC activity, however, have demonstrated potent therapeutic effects in animal models of arthritis and other chronic inflammatory diseases. In the present review we assess and reconcile these outwardly paradoxical study results to provide a working model for how alterations in HDAC activity may contribute to pathology in rheumatoid arthritis, and highlight key questions to be answered in the preclinical evaluation of compounds modulating these enzymes.
Figures

References
-
- Helmvan Mil van der, Wesoly JZ, Huizinga TWJ. Understanding the genetic contribution to rheumatoid arthritis. Curr Opin Rheumatol. 2005;17:299–304. doi: 10.1097/01.bor.0000160780.13012.be. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

