Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of varepsilonPKC and activation of aldehyde dehydrogenase 2
- PMID: 18983847
- PMCID: PMC2675554
- DOI: 10.1016/j.yjmcc.2008.09.713
Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of varepsilonPKC and activation of aldehyde dehydrogenase 2
Abstract
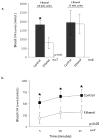
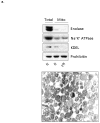
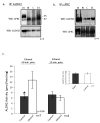
The cardioprotective effects of moderate alcohol consumption have been well documented in animal models and in humans. Protection afforded against ischemia and reperfusion injury (I/R) proceeds through an ischemic preconditioning-like mechanism involving the activation of epsilon protein kinase C (varepsilonPKC) and is dependent on the time and duration of ethanol treatment. However, the substrates of varepsilonPKC and the molecular mechanisms by which the enzyme protects the heart from oxidative damage induced by I/R are not fully described. Using an open-chest model of acute myocardial infarction in vivo, we find that intraperitoneal injection of ethanol (0.5 g/kg) 60 min prior to (but not 15 min prior to) a 30-minute transient ligation of the left anterior descending coronary artery reduced I/R-mediated injury by 57% (measured as a decrease of creatine phosphokinase release into the blood). Only under cardioprotective conditions, ethanol treatment resulted in the translocation of varepsilonPKC to cardiac mitochondria, where the enzyme bound aldehyde dehydrogenase-2 (ALDH2). ALDH2 is an intra-mitochondrial enzyme involved in the detoxification of toxic aldehydes such as 4-hydroxy-2-nonenal (4-HNE) and 4-HNE mediates oxidative damage, at least in part, by covalently modifying and inactivating proteins (by forming 4-HNE adducts). In hearts subjected to I/R after ethanol treatment, the levels of 4-HNE protein adducts were lower and JNK1/2 and ERK1/2 activities were diminished relative to the hearts from rats subjected to I/R in the absence of ethanol. Together, this work provides an insight into the mitochondrial-dependent basis of ethanol-induced and varepsilonPKC-mediated protection from cardiac ischemia, in vivo.
Figures



Similar articles
-
Activation of aldehyde dehydrogenase 2 (ALDH2) confers cardioprotection in protein kinase C epsilon (PKCvarepsilon) knockout mice.J Mol Cell Cardiol. 2010 Apr;48(4):757-64. doi: 10.1016/j.yjmcc.2009.10.030. Epub 2009 Nov 11. J Mol Cell Cardiol. 2010. PMID: 19913552 Free PMC article.
-
Alpha lipoic acid protects heart against myocardial ischemia-reperfusion injury through a mechanism involving aldehyde dehydrogenase 2 activation.Eur J Pharmacol. 2012 Mar 5;678(1-3):32-8. doi: 10.1016/j.ejphar.2011.12.042. Epub 2012 Jan 12. Eur J Pharmacol. 2012. PMID: 22266491
-
[Activation of mitochondrial aldehyde dehydrogenase 2 and inhibition of mitochondrial permeability transition pore involved in cardioprotection of ethanol postconditioning].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2010 Nov;39(6):566-71. doi: 10.3785/j.issn.1008-9292.2010.06.003. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2010. PMID: 21166048 Chinese.
-
Aldehyde dehydrogenase 2 in cardiac protection: a new therapeutic target?Trends Cardiovasc Med. 2009 Jul;19(5):158-64. doi: 10.1016/j.tcm.2009.09.003. Trends Cardiovasc Med. 2009. PMID: 20005475 Free PMC article. Review.
-
Salvaging the Ischemic Heart: Gi-Coupled Receptors in Mast Cells Activate a PKCε/ALDH2 Pathway Providing Anti-RAS Cardioprotection.Curr Med Chem. 2018;25(34):4416-4431. doi: 10.2174/0929867325666180214115127. Curr Med Chem. 2018. PMID: 29446730 Review.
Cited by
-
Aldehyde Dehydrogenase 2 as a Therapeutic Target in Oxidative Stress-Related Diseases: Post-Translational Modifications Deserve More Attention.Int J Mol Sci. 2022 Feb 28;23(5):2682. doi: 10.3390/ijms23052682. Int J Mol Sci. 2022. PMID: 35269824 Free PMC article. Review.
-
Discovery of a novel class of covalent inhibitor for aldehyde dehydrogenases.J Biol Chem. 2011 Dec 16;286(50):43486-94. doi: 10.1074/jbc.M111.293597. Epub 2011 Oct 21. J Biol Chem. 2011. PMID: 22021038 Free PMC article.
-
ALDH2 activator inhibits increased myocardial infarction injury by nitroglycerin tolerance.Sci Transl Med. 2011 Nov 2;3(107):107ra111. doi: 10.1126/scitranslmed.3002067. Sci Transl Med. 2011. PMID: 22049071 Free PMC article.
-
Estrogen receptor ERα plays a major role in ethanol-evoked myocardial oxidative stress and dysfunction in conscious female rats.Alcohol. 2016 Feb;50:27-35. doi: 10.1016/j.alcohol.2015.11.002. Epub 2015 Nov 26. Alcohol. 2016. PMID: 26695589 Free PMC article.
-
Mitochondrial energy metabolism disorder and apoptosis: a potential mechanism of postoperative ileus.Int J Clin Exp Med. 2015 Sep 15;8(9):14885-95. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26628970 Free PMC article.
References
-
- Gaziano JM, Gaziano TA, Glynn RJ, Sesso HD, Ajani UA, Stampfer MJ, et al. Light-to-moderate alcohol consumption and mortality in the Physicians’ Health Study enrollment cohort. Journal of the American College of Cardiology. 2000 Jan;35(1):96–105. - PubMed
-
- Renaud SC, Gueguen R, Schenker J, d’Houtaud A. Alcohol and mortality in middle-aged men from eastern France. Epidemiology. 1998 Mar;9(2):184–8. Cambridge, Mass. - PubMed
-
- Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991 Aug 24;338(8765):464–8. - PubMed
-
- Stampfer MJ, Colditz GA, Willett WC, Speizer FE, Hennekens CH. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. The New England journal of medicine. 1988 Aug 4;319(5):267–73. - PubMed
-
- Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW, Jr, et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. The New England journal of medicine. 1997 Dec 11;337(24):1705–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

