Cisplatin combined with zidovudine enhances cytotoxicity and oxidative stress in human head and neck cancer cells via a thiol-dependent mechanism
- PMID: 18983911
- PMCID: PMC2659778
- DOI: 10.1016/j.freeradbiomed.2008.10.023
Cisplatin combined with zidovudine enhances cytotoxicity and oxidative stress in human head and neck cancer cells via a thiol-dependent mechanism
Abstract
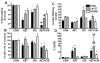
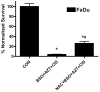
Oxidative stress and mitochondrial dysfunction in cancer cells represent features that may be exploited therapeutically. We determined whether agents that induce mitochondrial dysfunction, such as zidovudine (AZT) and cisplatin (CIS), could enhance killing of human head and neck cancer cells via oxidative stress. AZT- and/or CIS-induced cytotoxicity was determined using clonogenic survival, mitochondrial membrane potential was analyzed to investigate mitochondrial function, and glutathione was measured to determine thiol metabolism perturbations. AZT+CIS significantly increased toxicity and reduced mitochondrial membrane potential in FaDu, Cal-27, and SQ20B head and neck cancer cells while increasing the percentage of glutathione disulfide (%GSSG). Treatment with the thiol antioxidant N-acetylcysteine (NAC) reversed the loss of mitochondrial membrane potential and the increase in %GSSG and partially protected FaDu and Cal-27 cells from AZT+CIS. Finally, an inhibitor of glutathione synthesis, l-buthionine-[S,R]-sulfoximine, sensitized the cells to AZT+CIS-induced cytotoxicity, which was partially reversed by NAC. These results suggest that exposure of cancer cells to agents that induce mitochondrial dysfunction, such as AZT, causes significant sensitization to CIS-induced toxicity via disruptions in thiol metabolism and oxidative stress. These findings provide a biochemical rationale for evaluating agents that induce mitochondrial dysfunction in combination with chemotherapy and inhibitors of glutathione metabolism in head and neck cancer.
Figures
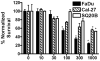


References
-
- Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979;39:1141–1149. - PubMed
-
- Konstantinov AA, Peskin AV, Popova E, Khomutov GB, Ruuge EK. Superoxide generation by the respiratory chain of tumor mitochondria. Biochim Biophys Acta. 1987;894:1–10. - PubMed
-
- Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. - PubMed
-
- Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. - PubMed
-
- Spitz DR, Sim JE, Ridnour LA, Galoforo SS, Lee YJ. Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Ann N Y Acad Sci. 2000;899:349–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

