Reciprocal modulation of function between the D1 and D2 dopamine receptors and the Na+,K+-ATPase
- PMID: 18984584
- PMCID: PMC2605984
- DOI: 10.1074/jbc.M805520200
Reciprocal modulation of function between the D1 and D2 dopamine receptors and the Na+,K+-ATPase
Abstract
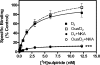
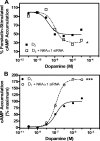
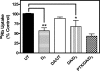
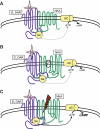
It is well documented that dopamine can increase or decrease the activity of the Na+,K+-ATPase (NKA, sodium pump) in an organ-specific fashion. This regulation can occur, at least partially, via receptor-mediated second messenger activation and can promote NKA insertion or removal from the plasma membrane. Using co-immunoprecipitation and mass spectrometry, we now show that, in both brain and HEK293T cells, D1 and D2 dopamine receptors (DARs) can exist in a complex with the sodium pump. To determine the impact of NKA on DAR function, biological assays were conducted with NKA and DARs co-expressed in HEK293T cells. In this system, expression of NKA dramatically decreased D1 and D2 DAR densities with a concomitant functional decrease in DAR-mediated regulation of cAMP levels. Interestingly, pharmacological inhibition of endogenous or overexpressed NKA enhanced DAR function without altering receptor number or localization. Similarly, DAR function was also augmented by small interfering RNA reduction of the endogenous NKA. These data suggest that, under basal conditions, NKA negatively regulates DAR function via protein-protein interactions. In reciprocal fashion, expression of DARs decreases endogenous NKA function in the absence of dopamine, implicating DAR proteins as regulators of NKA activity. Notably, dopamine stimulation or pertussis toxin inhibition of D2 receptor signaling did not alter NKA activity, indicating that the D2-mediated decrease in NKA function is dependent upon protein-protein interactions rather than signaling molecules. This evidence for reciprocal regulation between DARs and NKA provides a novel control mechanism for both DAR signaling and cellular ion balance.
Figures




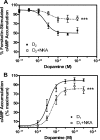
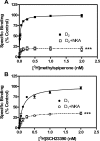
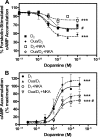


References
-
- El-Ghundi, M., O'Dowd, B. F., and George, S. R. (2007) Rev. Neurosci. 18 37-66 - PubMed
-
- Goldman-Rakic, P. S. (1998) Adv. Pharmacol. 42 707-711 - PubMed
-
- Missale, C., Nash, S. R., Robinson, S. W., Jaber, M., and Caron, M. G. (1998) Physiol. Rev. 78 189-225 - PubMed
-
- Schultz, W. (2002) Neuron 36 241-263 - PubMed
-
- Sealfon, S. C., and Olanow, C. W. (2000) Trends Neurosci. 23 S34-S40 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

