Effect of novel negative allosteric modulators of neuronal nicotinic receptors on cells expressing native and recombinant nicotinic receptors: implications for drug discovery
- PMID: 18984653
- PMCID: PMC2682284
- DOI: 10.1124/jpet.108.144576
Effect of novel negative allosteric modulators of neuronal nicotinic receptors on cells expressing native and recombinant nicotinic receptors: implications for drug discovery
Abstract
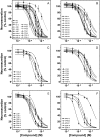
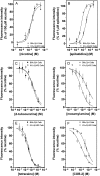
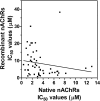
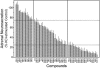
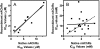
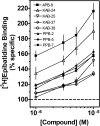
Allosteric modulation of nAChRs is considered to be one of the most promising approaches for drug design targeting nicotinic acetylcholine receptors (nAChRs). We have reported previously on the pharmacological activity of several compounds that seem to act noncompetitively to inhibit the activation of alpha3beta4(*) nAChRs. In this study, the effects of 51 structurally similar molecules on native and recombinant alpha3beta4 nAChRs are characterized. These 51 molecules inhibited adrenal neurosecretion activated via stimulation of native alpha3beta4(*) nAChR, with IC(50) values ranging from 0.4 to 13.0 microM. Using cells expressing recombinant alpha3beta4 nAChRs, these molecules inhibited calcium accumulation (a more direct assay to establish nAChR activity), with IC(50) values ranging from 0.7 to 38.2 microM. Radiolabeled nAChR binding studies to orthosteric sites showed no inhibitory activity on either native or recombinant nAChRs. Correlation analyses of the data from both functional assays suggested additional, non-nAChR activity of the molecules. To test this hypothesis, the effects of the drugs on neurosecretion stimulated through non-nAChR mechanisms were investigated; inhibitory effects ranged from no inhibition to 95% inhibition at concentrations of 10 microM. Correlation analyses of the functional data confirmed this hypothesis. Several of the molecules (24/51) increased agonist binding to native nAChRs, supporting allosteric interactions with nAChRs. Computational modeling and blind docking identified a binding site for our negative allosteric modulators near the orthosteric binding site of the receptor. In summary, this study identified several molecules for potential development as negative allosteric modulators and documented the importance of multiple screening assays for nAChR drug discovery.
Figures


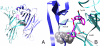
Similar articles
-
Negative allosteric modulators that target human alpha4beta2 neuronal nicotinic receptors.J Pharmacol Exp Ther. 2010 Sep 1;334(3):761-74. doi: 10.1124/jpet.110.168211. Epub 2010 Jun 15. J Pharmacol Exp Ther. 2010. PMID: 20551292 Free PMC article.
-
Receptor protection studies comparing recombinant and native nicotinic receptors: Evidence for a subpopulation of mecamylamine-sensitive native alpha3beta4* nicotinic receptors.Neurosci Lett. 2006 Jan 9;392(1-2):135-9. doi: 10.1016/j.neulet.2005.09.002. Epub 2005 Sep 29. Neurosci Lett. 2006. PMID: 16198480
-
Pharmacological and molecular studies on the interaction of varenicline with different nicotinic acetylcholine receptor subtypes. Potential mechanism underlying partial agonism at human α4β2 and α3β4 subtypes.Biochim Biophys Acta. 2015 Feb;1848(2):731-41. doi: 10.1016/j.bbamem.2014.11.003. Epub 2014 Dec 2. Biochim Biophys Acta. 2015. PMID: 25475645
-
Effects of neuronal nicotinic acetylcholine receptor allosteric modulators in animal behavior studies.Biochem Pharmacol. 2013 Oct 15;86(8):1054-62. doi: 10.1016/j.bcp.2013.05.018. Epub 2013 May 31. Biochem Pharmacol. 2013. PMID: 23732296 Free PMC article. Review.
-
Modes of Action, Resistance and Toxicity of Insecticides Targeting Nicotinic Acetylcholine Receptors.Curr Med Chem. 2017;24(27):2925-2934. doi: 10.2174/0929867324666170206142019. Curr Med Chem. 2017. PMID: 28176635 Review.
Cited by
-
Defining the putative inhibitory site for a selective negative allosteric modulator of human α4β2 neuronal nicotinic receptors.ACS Chem Neurosci. 2012 Sep 19;3(9):682-92. doi: 10.1021/cn300035f. Epub 2012 May 25. ACS Chem Neurosci. 2012. PMID: 23019495 Free PMC article.
-
Discovery of benzamide analogs as negative allosteric modulators of human neuronal nicotinic receptors: pharmacophore modeling and structure-activity relationship studies.Bioorg Med Chem. 2013 Aug 1;21(15):4730-43. doi: 10.1016/j.bmc.2013.03.082. Epub 2013 Apr 6. Bioorg Med Chem. 2013. PMID: 23757208 Free PMC article.
-
Identifying the binding site of novel methyllycaconitine (MLA) analogs at α4β2 nicotinic acetylcholine receptors.ACS Chem Neurosci. 2010 Dec 15;1(12):796-809. doi: 10.1021/cn100073x. Epub 2010 Oct 7. ACS Chem Neurosci. 2010. PMID: 22778816 Free PMC article.
-
Structure-activity relationship studies of sulfonylpiperazine analogues as novel negative allosteric modulators of human neuronal nicotinic receptors.J Med Chem. 2011 Dec 22;54(24):8681-92. doi: 10.1021/jm201294r. Epub 2011 Nov 18. J Med Chem. 2011. PMID: 22060139 Free PMC article.
-
3D-QSAR and 3D-QSSR models of negative allosteric modulators facilitate the design of a novel selective antagonist of human α4β2 neuronal nicotinic acetylcholine receptors.Bioorg Med Chem Lett. 2012 Feb 15;22(4):1797-813. doi: 10.1016/j.bmcl.2011.11.051. Epub 2011 Nov 20. Bioorg Med Chem Lett. 2012. PMID: 22285942 Free PMC article.
References
-
- Adam LP and Henderson EG (1990) Calcium channel effectors are potent noncompetitive blockers of acetylcholine receptors. Pflugers Arch 416 586-593. - PubMed
-
- Alés E, Gullo F, Arias E, Olivares R, García AG, Wanke E, and López MG (2006) Blockade of Ca2+-activated K+ channels by galantamine can also contribute to the potentiation of catecholamine secretion from chromaffin cells. Eur J Pharmacol 548 45-52. - PubMed
-
- Arias HR, Bhumireddy P, and Bouzat C (2006) Molecular mechanisms and binding site locations for noncompetitive antagonists of nicotinic acetylcholine receptors. Int J Biochem Cell Biol 38 1254-1276. - PubMed
-
- Bergmeier SC, Ismail KA, Arason KM, McKay S, Bryant DL, and McKay DB (2004) Structure activity studies of ring E analogues of methyllycaconitine. Part 2: synthesis of antagonists to the α3β4 nicotinic acetylcholine receptors through modifications to the ester. Bioorg Med Chem Lett 14 3739-3742. - PubMed
-
- Bergmeier SC, Lapinsky DJ, Free RB, and McKay DB (1999) Ring E analogs of methyllycaconitine (MLA) as novel nicotinic antagonists. Bioorg Med Chem Lett 9 2263-2266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

