Differential gene expression in a coculture model of angiogenesis reveals modulation of select pathways and a role for Notch signaling
- PMID: 18984672
- PMCID: PMC2636923
- DOI: 10.1152/physiolgenomics.90318.2008
Differential gene expression in a coculture model of angiogenesis reveals modulation of select pathways and a role for Notch signaling
Abstract
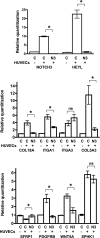
Communication between endothelial and mural cells (smooth muscle cells, pericytes, and fibroblasts) can dictate blood vessel size and shape during angiogenesis, and control the functional aspects of mature blood vessels, by determining things such as contractile properties. The ability of these different cell types to regulate each other's activities led us to ask how their interactions directly modulate gene expression. To address this, we utilized a three-dimensional model of angiogenesis and screened for genes whose expression was altered under coculture conditions. Using a BeadChip array, we identified 323 genes that were uniquely regulated when endothelial cells and mural cells (fibroblasts) were cultured together. Data mining tools revealed that differential expression of genes from the integrin, blood coagulation, and angiogenesis pathways were overrepresented in coculture conditions. Scans of the promoters of these differentially modulated genes identified a multitude of conserved C promoter binding factor (CBF)1/CSL elements, implicating Notch signaling in their regulation. Accordingly, inhibition of the Notch pathway with gamma-secretase inhibitor DAPT or NOTCH3-specific small interfering RNA blocked the coculture-induced regulation of several of these genes in fibroblasts. These data show that coculturing of endothelial cells and fibroblasts causes profound changes in gene expression and suggest that Notch signaling is a critical mediator of the resultant transcription.
Figures



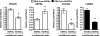

Similar articles
-
NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1.Circ Res. 2009 Feb 27;104(4):466-75. doi: 10.1161/CIRCRESAHA.108.184846. Epub 2009 Jan 15. Circ Res. 2009. PMID: 19150886 Free PMC article.
-
Endothelial cells downregulate apolipoprotein D expression in mural cells through paracrine secretion and Notch signaling.Am J Physiol Heart Circ Physiol. 2011 Sep;301(3):H784-93. doi: 10.1152/ajpheart.00116.2011. Epub 2011 Jun 24. Am J Physiol Heart Circ Physiol. 2011. PMID: 21705670 Free PMC article.
-
Type IV collagen expression is regulated by Notch3-mediated Notch signaling during angiogenesis.Biochem Biophys Res Commun. 2025 Feb 16;749:151351. doi: 10.1016/j.bbrc.2025.151351. Epub 2025 Jan 16. Biochem Biophys Res Commun. 2025. PMID: 39842335
-
Notch-mediated cellular interactions between vascular cells.Curr Opin Cell Biol. 2023 Dec;85:102254. doi: 10.1016/j.ceb.2023.102254. Epub 2023 Oct 11. Curr Opin Cell Biol. 2023. PMID: 37832167 Review.
-
Roles of Ubiquitination and SUMOylation in the Regulation of Angiogenesis.Curr Issues Mol Biol. 2020;35:109-126. doi: 10.21775/cimb.035.109. Epub 2019 Aug 18. Curr Issues Mol Biol. 2020. PMID: 31422936 Review.
Cited by
-
Myoendothelial Junctions of Mature Coronary Vessels Express Notch Signaling Proteins.Front Physiol. 2020 Feb 4;11:29. doi: 10.3389/fphys.2020.00029. eCollection 2020. Front Physiol. 2020. PMID: 32116749 Free PMC article.
-
Evaluation of Notch3 Deficiency in Diabetes-Induced Pericyte Loss in the Retina.J Vasc Res. 2018;55(5):308-318. doi: 10.1159/000493151. Epub 2018 Oct 22. J Vasc Res. 2018. PMID: 30347392 Free PMC article.
-
Impaired endothelium-mesenchymal stem cells cross-talk in systemic sclerosis: a link between vascular and fibrotic features.Arthritis Res Ther. 2014 Sep 24;16(5):442. doi: 10.1186/s13075-014-0442-z. Arthritis Res Ther. 2014. PMID: 25248297 Free PMC article.
-
The cellular Notch1 protein promotes KSHV reactivation in an Rta-dependent manner.J Virol. 2024 Aug 20;98(8):e0078824. doi: 10.1128/jvi.00788-24. Epub 2024 Jul 8. J Virol. 2024. PMID: 38975769 Free PMC article.
-
Notch3 directs differentiation of brain mural cells from human pluripotent stem cell-derived neural crest.Sci Adv. 2024 Feb 2;10(5):eadi1737. doi: 10.1126/sciadv.adi1737. Epub 2024 Feb 2. Sci Adv. 2024. PMID: 38306433 Free PMC article.
References
-
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 97: 512–523, 2005. - PubMed
-
- Bray SJ Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7: 678–689, 2006. - PubMed
-
- Carmeliet P Angiogenesis in life, disease and medicine. Nature 438: 932–936, 2005. - PubMed
-
- Chand HS, Foster DC, Kisiel W. Structure, function and biology of tissue factor pathway inhibitor-2. Thromb Haemost 94: 1122–1130, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

