Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function
- PMID: 18984743
- PMCID: PMC2628614
- DOI: 10.2337/db08-0792
Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function
Abstract
Objective: Somatostatin (SST) is secreted by islet delta-cells and by extraislet neuroendocrine cells. SST receptors have been identified on alpha- and beta-cells, and exogenous SST inhibits insulin and glucagon secretion, consistent with a role for SST in regulating alpha- and beta-cell function. However, the specific intraislet function of delta-cell SST remains uncertain. We have used Sst(-/-) mice to investigate the role of delta-cell SST in the regulation of insulin and glucagon secretion in vitro and in vivo.
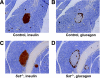
Research design and methods: Islet morphology was assessed by histological analysis. Hormone levels were measured by radioimmunoassay in control and Sst(-/-) mice in vivo and from isolated islets in vitro.
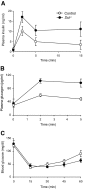
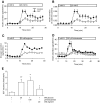
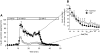
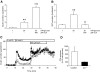
Results: Islet size and organization did not differ between Sst(-/-) and control islets, nor did islet glucagon or insulin content. Sst(-/-) mice showed enhanced insulin and glucagon secretory responses in vivo. In vitro stimulus-induced insulin and glucagon secretion was enhanced from perifused Sst(-/-) islets compared with control islets and was inhibited by exogenous SST in Sst(-/-) but not control islets. No difference in the switch-off rate of glucose-stimulated insulin secretion was observed between genotypes, but the cholinergic agonist carbamylcholine enhanced glucose-induced insulin secretion to a lesser extent in Sst(-/-) islets compared with controls. Glucose suppressed glucagon secretion from control but not Sst(-/-) islets.
Conclusions: We suggest that delta-cell SST exerts a tonic inhibitory influence on insulin and glucagon secretion, which may facilitate the islet response to cholinergic activation. In addition, delta-cell SST is implicated in the nutrient-induced suppression of glucagon secretion.
Figures


Comment in
-
Regulating glucagon secretion: somatostatin in the spotlight.Diabetes. 2009 Feb;58(2):299-301. doi: 10.2337/db08-1534. Diabetes. 2009. PMID: 19171745 Free PMC article. No abstract available.
References
-
- Vieira E, Salehi A, Gylfe E: Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia 50: 370–379, 2007 - PubMed
-
- Bishop AE, Polak JM: The anatomy, organization and ultrastructure of the islets of Langerhans. In Textbook of Diabetes. Pickup JC, Williams G, Eds. Oxford, U.K., Blackwell Science, 2002, p. 10.1–10.6
-
- Halban PA, Wollheim CB, Blondel B, Meda P, Niesor EN, Mintz DH: The possible importance of contact between pancreatic islet cells for the control of insulin release. Endocrinology 111: 86–94, 1982 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

