Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease
- PMID: 18984744
- PMCID: PMC2628604
- DOI: 10.2337/db08-0799
Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease
Abstract
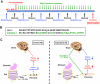
Objective: The aim of this study was to find an effective treatment for the genetic form of diabetes that is present in some Huntington's disease patients and in Huntington's disease mouse models. Huntington's disease is a neurodegenerative disorder caused by a polyglutamine expansion within the huntingtin protein. Huntington's disease patients exhibit neuronal dysfunction/degeneration, chorea, and progressive weight loss. Additionally, they suffer from abnormalities in energy metabolism affecting both the brain and periphery. Similarly to Huntington's disease patients, mice expressing the mutated human huntingtin protein also exhibit neurodegenerative changes, motor dysfunction, perturbed energy metabolism, and elevated blood glucose levels.
Research design and methods: Huntington's disease mice were treated with an FDA-approved antidiabetic glucagon-like peptide 1 receptor agonist, exendin-4 (Ex-4), to test whether euglycemia could be achieved, whether pancreatic dysfunction could be alleviated, and whether the mice showed any neurological benefit. Blood glucose and insulin levels and various appetite hormone concentrations were measured during the study. Additionally, motor performance and life span were quantified and mutant huntingtin (mhtt) aggregates were measured in both the pancreas and brain.
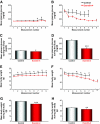
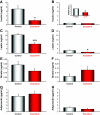
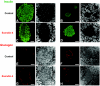
Results: Ex-4 treatment ameliorated abnormalities in peripheral glucose regulation and suppressed cellular pathology in both brain and pancreas in a mouse model of Huntington's disease. The treatment also improved motor function and extended the survival time of the Huntington's disease mice. These clinical improvements were correlated with reduced accumulation of mhtt protein aggregates in both islet and brain cells.
Conclusions: Targeting both peripheral and neuronal deficits, Ex-4 is an attractive agent for therapeutic intervention in Huntington's disease patients suffering from diabetes.
Figures

Similar articles
-
Huntington's disease of the endocrine pancreas: insulin deficiency and diabetes mellitus due to impaired insulin gene expression.Neurobiol Dis. 2002 Dec;11(3):410-24. doi: 10.1006/nbdi.2002.0562. Neurobiol Dis. 2002. PMID: 12586550
-
IGF-1 protects against diabetic features in an in vivo model of Huntington's disease.Exp Neurol. 2011 Oct;231(2):314-9. doi: 10.1016/j.expneurol.2011.06.016. Epub 2011 Jul 7. Exp Neurol. 2011. PMID: 21763311
-
Passive immunization against phosphorylated tau improves features of Huntington's disease pathology.Mol Ther. 2022 Apr 6;30(4):1500-1522. doi: 10.1016/j.ymthe.2022.01.020. Epub 2022 Jan 17. Mol Ther. 2022. PMID: 35051614 Free PMC article.
-
Dysregulation of synaptic proteins, dendritic spine abnormalities and pathological plasticity of synapses as experience-dependent mediators of cognitive and psychiatric symptoms in Huntington's disease.Neuroscience. 2013 Oct 22;251:66-74. doi: 10.1016/j.neuroscience.2012.05.043. Epub 2012 May 24. Neuroscience. 2013. PMID: 22633949 Review.
-
Large Animal Models of Huntington's Disease.Curr Top Behav Neurosci. 2015;22:149-60. doi: 10.1007/7854_2013_246. Curr Top Behav Neurosci. 2015. PMID: 24048953 Free PMC article. Review.
Cited by
-
Neuroprotective effects of exendin-4 in rat model of spinal cord injury via inhibiting mitochondrial apoptotic pathway.Int J Clin Exp Pathol. 2015 May 1;8(5):4837-43. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26191175 Free PMC article.
-
Mouse models of polyglutamine diseases: review and data table. Part I.Mol Neurobiol. 2012 Oct;46(2):393-429. doi: 10.1007/s12035-012-8315-4. Epub 2012 Sep 7. Mol Neurobiol. 2012. PMID: 22956270 Free PMC article. Review.
-
Expression of mutant huntingtin in leptin receptor-expressing neurons does not control the metabolic and psychiatric phenotype of the BACHD mouse.PLoS One. 2012;7(12):e51168. doi: 10.1371/journal.pone.0051168. Epub 2012 Dec 10. PLoS One. 2012. PMID: 23251447 Free PMC article.
-
Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders.Exp Gerontol. 2009 Oct;44(10):625-33. doi: 10.1016/j.exger.2009.07.003. Epub 2009 Jul 19. Exp Gerontol. 2009. PMID: 19622391 Free PMC article. Review.
-
Allosteric modulators of g protein-coupled receptors: future therapeutics for complex physiological disorders.J Pharmacol Exp Ther. 2009 Nov;331(2):340-8. doi: 10.1124/jpet.109.156380. Epub 2009 Aug 10. J Pharmacol Exp Ther. 2009. PMID: 19667132 Free PMC article. Review.
References
-
- Andrew SE, Goldberg YP, Kremer B, Telenius H, Theilmann J, Adam S, Starr E, Squitieri F, Lin B, Kalchman MA, Graham RK, Hayden MR: The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nat Genet 4: 398–403, 1993 - PubMed
-
- Bates G: Huntingtin aggregation and toxicity in Huntington's disease. Lancet 361: 1642–1644, 2003 - PubMed
-
- Aziz NA, Swaab DF, Pijl H, Roos RA: Hypothalamic dysfunction and neuroendocrine and metabolic alterations in Huntington's disease: clinical consequences and therapeutic implications. Rev Neurosci 18: 223–251, 2007 - PubMed
-
- Gaba AM, Zhang K, Marder K, Moskowitz CB, Werner P, Boozer CN: Energy balance in early-stage Huntington disease. Am J Clin Nutr 81: 1335–1341, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

