Co-localization and interaction of human organic anion transporter 4 with caveolin-1 in primary cultured human placental trophoblasts
- PMID: 18985008
- PMCID: PMC2679353
- DOI: 10.3858/emm.2008.40.5.505
Co-localization and interaction of human organic anion transporter 4 with caveolin-1 in primary cultured human placental trophoblasts
Abstract
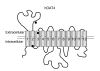
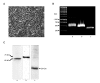
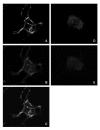
The human organic anion transporter 4 (hOAT4) has been identified as the fourth isoform of OAT family. hOAT4 contributes to move several negatively charged organic compounds between cells and their extracellular milieu. The functional characteristics and regulatory mechanisms of hOAT4 remain to be elucidated. It is well known that caveolin plays a role in modulating proteins having some biological functions. To address this issue, we investigated the co-localization and interaction between hOAT4 and caveolin-1. hOAT4 and caveolin-1 (mRNA and protein expression) were observed in cultured human placental trophoblasts isolated from placenta. The confocal microscopy of immuno-cytochemistry using primary cultured human trophoblasts showed hOAT4 and caveolin-1 were co-localized at the plasma membrane of the cell. This finding was confirmed by Western blot analysis using isolated caveolae-enriched membrane fractions and immune-precipitates from the trophoblasts. When synthesized cRNA of hOAT4 along with scrambled- or antisense-oligodeoxynucleotide (ODN) of Xenopus caveolin-1 were co-injected to Xenopus oocytes, the [3H]estrone sulfate uptake was significantly decreased by the co-injection of antisense ODN but not by scrambled ODN. These findings suggest that hOAT4 and caveolin-1 share a cellular expression in the plasma membrane and caveolin-1 up-regulates the organic anionic compound uptake by hOAT4 under the normal physiological condition.
Figures
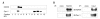
Similar articles
-
Evidence for rat organic anion transporter 3 association with caveolin-1 in rat kidney.IUBMB Life. 2005 Feb;57(2):109-17. doi: 10.1080/15216540500104750. IUBMB Life. 2005. PMID: 16036570
-
Co-localization and interaction of organic anion transporter 1 with caveolin-2 in rat kidney.Exp Mol Med. 2005 Jun 30;37(3):204-12. doi: 10.1038/emm.2005.28. Exp Mol Med. 2005. PMID: 16000875
-
Co-localization and interaction of b0,+-type amino acid transporter 1 (BAT1) with caveolin-1 in rat kidney.J Nephrol. 2005 Nov-Dec;18(6):681-9. J Nephrol. 2005. PMID: 16358225
-
Introduction of Organic Anion Transporters (SLC22A) and a Regulatory Mechanism by Caveolins.Electrolyte Blood Press. 2006 Mar;4(1):8-17. doi: 10.5049/EBP.2006.4.1.8. Electrolyte Blood Press. 2006. PMID: 24459480 Free PMC article. Review.
-
Human placental trophoblast culture: one-sided and two-sided models.Proc Nutr Soc. 1991 Aug;50(2):349-54. doi: 10.1079/pns19910045. Proc Nutr Soc. 1991. PMID: 1749802 Review. No abstract available.
Cited by
-
Evaluating caveolin interactions: do proteins interact with the caveolin scaffolding domain through a widespread aromatic residue-rich motif?PLoS One. 2012;7(9):e44879. doi: 10.1371/journal.pone.0044879. Epub 2012 Sep 17. PLoS One. 2012. PMID: 23028656 Free PMC article.
-
Oligomerization of drug transporters: Forms, functions, and mechanisms.Acta Pharm Sin B. 2024 May;14(5):1924-1938. doi: 10.1016/j.apsb.2024.01.007. Epub 2024 Jan 20. Acta Pharm Sin B. 2024. PMID: 38799641 Free PMC article. Review.
-
Short-term regulation of organic anion transporters.Pharmacol Ther. 2010 Jan;125(1):55-61. doi: 10.1016/j.pharmthera.2009.08.002. Epub 2009 Sep 8. Pharmacol Ther. 2010. PMID: 19744520 Free PMC article. Review.
-
Vitamin D Reduces Oxidative Stress-Induced Procaspase-3/ROCK1 Activation and MP Release by Placental Trophoblasts.J Clin Endocrinol Metab. 2017 Jun 1;102(6):2100-2110. doi: 10.1210/jc.2016-3753. J Clin Endocrinol Metab. 2017. PMID: 28368445 Free PMC article.
-
Trafficking and other regulatory mechanisms for organic anion transporting polypeptides and organic anion transporters that modulate cellular drug and xenobiotic influx and that are dysregulated in disease.Br J Pharmacol. 2017 Jul;174(13):1908-1924. doi: 10.1111/bph.13785. Epub 2017 Apr 24. Br J Pharmacol. 2017. PMID: 28299773 Free PMC article. Review.
References
-
- Bischof P, Truong K, Campana A. Regulation of Trophoblastic Gelatinases by Proto-oncogenes. Placenta. 2003;24:155–163. - PubMed
-
- Cai C, Zhu H, Chen J. Overexpression of caveolin-1 increases plasma membrane fluidity and reduces P-glycoprotein function in Hs578T/Dox. Biochem Biophys Res Commun. 2004;320:868–874. - PubMed
-
- Cha SH, Sekine T, Fukushima JI, Kanai Y, Kobayashi Y, Goya T, Endou H. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001;59:1277–1286. - PubMed
-
- Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem. 2000;275:4507–4512. - PubMed
-
- Cha SH, Shin SY, Jung SY, Kim YT, Park YJ, Kwak JO, Kim HW, Suh CK. Evidence for Na+/Ca2+ exchanger 1 association with caveolin-1 and -2 in C6 glioma cells. IUBMB Life. 2004;56:621–627. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
