pH-sensitive Photoluminescence of CdSe/ZnSe/ZnS Quantum Dots in Human Ovarian Cancer Cells
- PMID: 18985164
- PMCID: PMC2577287
- DOI: 10.1021/jp0654718
pH-sensitive Photoluminescence of CdSe/ZnSe/ZnS Quantum Dots in Human Ovarian Cancer Cells
Abstract
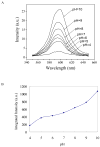
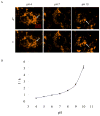
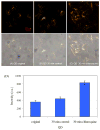
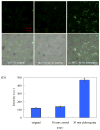
The photoluminescence of mercaptoacetic acid (MAA)-capped CdSe/ZnSe/ZnS semiconductor nanocrystal quantum dots (QDs) in SKOV-3 human ovarian cancer cells is pH-dependent, suggesting applications in which QDs serve as intracellular pH sensors. In both fixed and living cells the fluorescence intensity of intracellular MAA-capped QDs (MAA QDs) increases monotonically with increasing pH. The electrophoretic mobility of MAA QDs also increases with pH, indicating an association between surface charging and fluorescence emission. MAA dissociates from the ZnS outer shell at low pH, resulting in aggregation and loss of solubility, and this may also contribute to the MAA QD fluorescence changes observed in the intracellular environment.
Figures

References
-
- Liu JW, Lu Y. J Am Chem Soc. 2003;125:6642–6643. - PubMed
-
- Diéguez A, Romano-Rodriguez A, Morante JR, Kappler J, Bârsan N, Göpel W. Sensors and Actuators B. 1999;60:125–137.
-
- Liang JG, Ai XP, He ZK, Pang DW. Analyst. 2004;129:619–622. - PubMed
-
- Mekis I, Talapin DV, Kornowski A, Haase M, Weller H. J Phys Chem B. 2003;107:7454–7462.
-
- Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, Heine JR, Mattoussi H, Ober R, Jensen KF, Bawendi MG. J Phys Chem B. 1997;101:9463–9475.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
