Fundamentals of traveling wave ion mobility spectrometry
- PMID: 18986171
- PMCID: PMC2761765
- DOI: 10.1021/ac8016295
Fundamentals of traveling wave ion mobility spectrometry
Abstract
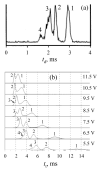
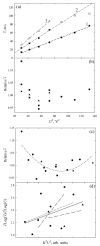
Traveling wave ion mobility spectrometry (TW IMS) is a new IMS method implemented in the Synapt IMS/mass spectrometry system (Waters). Despite its wide adoption, the foundations of TW IMS were only qualitatively understood and factors governing the ion transit time (the separation parameter) and resolution remained murky. Here we develop the theory of TW IMS using derivations and ion dynamics simulations. The key parameter is the ratio (c) of ion drift velocity at the steepest wave slope to wave speed. At low c, the ion transit velocity is proportional to the squares of mobility (K) and electric field intensity (E), as opposed to linear scaling in drift tube (DT) IMS and differential mobility analyzers. At higher c, the scaling deviates from quadratic in a way controlled by the waveform profile, becoming more gradual with the ideal triangular profile but first steeper and then more gradual for realistic profiles with variable E. At highest c, the transit velocity asymptotically approaches the wave speed. Unlike with DT IMS, the resolving power of TW IMS depends on mobility, scaling as K(1/2) in the low-c limit and less at higher c. A nonlinear dependence of the transit time on mobility means that the true resolving power of TW IMS differs from that indicated by the spectrum. A near-optimum resolution is achievable over an approximately 300-400% range of mobilities. The major predicted trends are in agreement with TW IMS measurements for peptide ions as a function of mobility, wave amplitude, and gas pressure. The issues of proper TW IMS calibration and ion distortion by field heating are also discussed. The new quantitative understanding of TW IMS separations allows rational optimization of instrument design and operation and improved spectral calibration.
Figures







References
-
- Eiceman GA, Karpas Z. Ion Mobility Spectrometry. CRC Press; Boca Raton: 2004.
-
- Hill HH, Siems WF, Louis RH, McMinn DG. Anal. Chem. 1990;62:A1201. - PubMed
-
- Hoaglund-Hyzer CS, Counterman AE, Clemmer DE. Chem. Rev. 1999;99:3037. - PubMed
-
- Shvartsburg AA, Hudgins RR, Dugourd P, Jarrold MF. Chem. Soc. Rev. 2001;30:26.
-
- Taraszka JA, Gao X, Valentine SJ, Sowell RA, Koeniger SL, Miller DF, Kaufman TC, Clemmer DE. J. Proteome Res. 2005;4:1238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

