A metapopulation modelling framework for gonorrhoea and other sexually transmitted infections in heterosexual populations
- PMID: 18986961
- PMCID: PMC2855508
- DOI: 10.1098/rsif.2008.0394
A metapopulation modelling framework for gonorrhoea and other sexually transmitted infections in heterosexual populations
Abstract
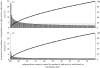
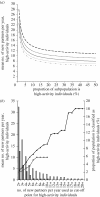
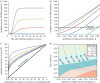
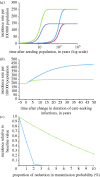
Gonorrhoea continues to be a public health problem in the UK, and is the second most common bacterial sexually transmitted infection (STI) after chlamydia. In the UK, gonorrhoea is disproportionately concentrated in epidemiologically distinct subpopulations, with much higher incidence rates in young people, some ethnic minorities and inner city subpopulations. The original model of STI transmission proposed by Hethcote and Yorke explained some of these features through the concept of the 'core group'. Since then, several authors have modified the original model approach to include multiple sexual activity classes, but found this modelling approach to be inadequate when applied to low-prevalence settings such as the UK. We present a metapopulation framework for modelling gonorrhoea and other STIs. The model proposes that the epidemiology of gonorrhoea is largely driven by subpopulations with higher than average concentrations of individuals with high sexual risk activity. We show how this conceptualization of gonococcal epidemiology overcomes key limitations associated with some of the prior efforts to model gonorrhoea. We also use the model to explain several epidemiological features of gonorrhoea, such as its asymmetric distribution across subpopulations, and the contextual risk experienced by members of at-risk subpopulations. Finally, we extend the model to explain the distribution of other STIs, using chlamydia as an example of a more ubiquitous bacterial STI.
Figures

References
-
- Becker K. M., Glass G. E., Brathwaite W., Zenilman J. M. 1998. Geographic epidemiology of gonorrhea in Baltimore, Maryland, using a geographic information system. Am. J. Epidemiol. 147, 709–716. - PubMed
-
- Brunham R. C., Plummer F. A. 1990. A general model of sexually transmitted disease epidemiology and its implications for control. Med. Clin. North Am. 74, 1339–1352. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
