RecG interacts directly with SSB: implications for stalled replication fork regression
- PMID: 18986999
- PMCID: PMC2602778
- DOI: 10.1093/nar/gkn795
RecG interacts directly with SSB: implications for stalled replication fork regression
Abstract
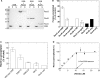
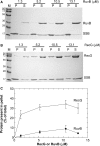
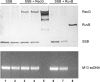
RecG and RuvAB are proposed to act at stalled DNA replication forks to facilitate replication restart. To define the roles of these proteins in fork regression, we used a combination of assays to determine whether RecG, RuvAB or both are capable of acting at a stalled fork. The results show that RecG binds to the C-terminus of single-stranded DNA binding protein (SSB) forming a stoichiometric complex of 2 RecG monomers per SSB tetramer. This binding occurs in solution and to SSB protein bound to single stranded DNA (ssDNA). The result of this binding is stabilization of the interaction of RecG with ssDNA. In contrast, RuvAB does not bind to SSB. Side-by-side analysis of the catalytic efficiency of the ATPase activity of each enzyme revealed that (-)scDNA and ssDNA are potent stimulators of the ATPase activity of RecG but not for RuvAB, whereas relaxed circular DNA is a poor cofactor for RecG but an excellent one for RuvAB. Collectively, these data suggest that the timing of repair protein access to the DNA at stalled forks is determined by the nature of the DNA available at the fork. We propose that RecG acts first, with RuvAB acting either after RecG or in a separate pathway following protein-independent fork regression.
Figures



References
-
- Kowalczykowski SC. Initiation of genetic recombination and recombination-dependent replication. Trends Biochem. Sci. 2000;25:156–165. - PubMed
-
- Seigneur M, Bidnenko V, Ehrlich SD, Michel B. RuvAB acts at arrested replication forks. Cell. 1998;95:419–430. - PubMed
-
- Cox MM, Goodman MF, Kreuzer KN, Sherratt DJ, Sandler SJ, Marians KJ. The importance of repairing stalled replication forks. Nature. 2000;404:37–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

