Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection
- PMID: 18987148
- PMCID: PMC2612355
- DOI: 10.1128/JVI.02036-08
Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection
Erratum in
- J Virol. 2009 May;83(9):4713-5
Abstract
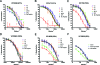
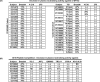
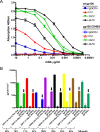
The characterization of the cross-reactive, or heterologous, neutralizing antibody responses developed during human immunodeficiency virus type 1 (HIV-1) infection and the identification of factors associated with their generation are relevant to the development of an HIV vaccine. We report that in healthy HIV-positive, antiretroviral-naïve subjects, the breadth of plasma heterologous neutralizing antibody responses correlates with the time since infection, plasma viremia levels, and the binding avidity of anti-Env antibodies. Anti-CD4-binding site antibodies are responsible for the exceptionally broad cross-neutralizing antibody responses recorded only in rare plasma samples. However, in most cases examined, antibodies to the variable regions and to the CD4-binding site of Env modestly contributed in defining the overall breadth of these responses. Plasmas with broad cross-neutralizing antibody responses were identified that targeted the gp120 subunit, but their precise epitopes mapped outside the variable regions and the CD4-binding site. Finally, although several plasmas were identified with cross-neutralizing antibody responses that were not directed against gp120, only one plasma with a moderate breadth of heterologous neutralizing antibody responses contained cross-reactive neutralizing antibodies against the 4E10 epitope, which is within the gp41 transmembrane subunit. Overall, our study indicates that more than one pathway leads to the development of broad cross-reactive neutralizing antibodies during HIV infection and that the virus continuously escapes their action.
Figures




References
-
- Backliwal, G., M. Hildinger, V. Hasija, and F. M. Wurm. 2008. High-density transfection with HEK-293 cells allows doubling of transient titers and removes need for a priori DNA complex formation with PEI. Biotechnol. Bioeng. 99721-727. - PubMed
-
- Binley, J. M., E. A. Lybarger, E. T. Crooks, M. S. Seaman, E. Gray, K. L. Davis, J. M. Decker, D. Wycuff, L. Harris, N. Hawkins, B. Wood, C. Nathe, D. Richman, G. D. Tomaras, F. Bibollet-Ruche, J. E. Robinson, L. Morris, G. M. Shaw, D. C. Montefiori, and J. R. Mascola. 24 September 2008. Profiling the specificity of neutralizing antibodies in a large panel of HIV-1 plasmas from subtype B and C chronic infections. J. Virol. 8211651-11668. - PMC - PubMed
-
- Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 7813232-13252. - PMC - PubMed
-
- Blish, C. A., R. Nedellec, K. Mandaliya, D. E. Mosier, and J. Overbaugh. 2007. HIV-1 subtype A envelope variants from early in infection have variable sensitivity to neutralization and to inhibitors of viral entry. AIDS 21693-702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

