Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels
- PMID: 18987181
- PMCID: PMC6671315
- DOI: 10.1523/JNEUROSCI.1972-08.2008
Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels
Abstract
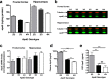
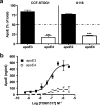
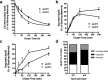
Inheritance of the apoE4 allele (epsilon4) increases the risk of developing Alzheimer's disease; however, the mechanisms underlying this association remain elusive. Recent data suggest that inheritance of epsilon4 may lead to reduced apoE protein levels in the CNS. We therefore examined apoE protein levels in the brains, CSF and plasma of epsilon2/2, epsilon3/3, and epsilon4/4 targeted replacement mice. These apoE mice showed a genotype-dependent decrease in apoE levels; epsilon2/2 >epsilon3/3 >epsilon4/4. Next, we sought to examine the relative contributions of apoE4 and apoE3 in the epsilon3/4 mouse brains. ApoE4 represented 30-40% of the total apoE. Moreover, the absolute amount of apoE3 per allele was similar between epsilon3/3 and epsilon3/4 mice, implying that the reduced levels of total apoE in epsilon3/4 mice can be explained by the reduction in apoE4 levels. In culture medium from epsilon3/4 human astrocytoma or epsilon3/3, epsilon4/4 and epsilon3/4 primary astrocytes, apoE4 levels were consistently lower than apoE3. Secreted cholesterol levels were also lower from epsilon4/4 astrocytes. Pulse-chase experiments showed an enhanced degradation and reduced half-life of newly synthesized apoE4 compared with apoE3. Together, these data suggest that astrocytes preferentially degrade apoE4, leading to reduced apoE4 secretion and ultimately to reduced brain apoE levels. Moreover, the genotype-dependent decrease in CNS apoE levels, mirror the relative risk of developing AD, and suggest that low levels of total apoE exhibited by epsilon4 carriers may directly contribute to the disease progression, perhaps by reducing the capacity of apoE to promote synaptic repair and/or Abeta clearance.
Figures



References
-
- Beffert U, Cohn JS, Petit-Turcotte C, Tremblay M, Aumont N, Ramassamy C, Davignon J, Poirier J. Apolipoprotein E and beta-amyloid levels in the hippocampus and frontal cortex of Alzheimer's disease subjects are disease-related and apolipoprotein E genotype dependent. Brain Res. 1999;843:87–94. - PubMed
-
- Bertrand P, Poirier J, Oda T, Finch CE, Pasinetti GM. Association of apolipoprotein E genotype with brain levels of apolipoprotein E and apolipoprotein J (clusterin) in Alzheimer disease. Brain Res Mol Brain Res. 1995;33:174–178. - PubMed
-
- Burns MP, Vardanian L, Pajoohesh-Ganji A, Wang L, Cooper M, Harris DC, Duff K, Rebeck GW. The effects of ABCA1 on cholesterol efflux and Abeta levels in vitro and in vivo. J Neurochem. 2006;98:792–800. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
