Glial chain migration requires pioneer cells
- PMID: 18987199
- PMCID: PMC6671288
- DOI: 10.1523/JNEUROSCI.1361-08.2008
Glial chain migration requires pioneer cells
Abstract
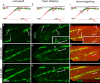
The migration of glial chains along the nerve entails directional and coordinated movement. Despite its importance in the formation of the nervous system, this process remains poorly understood, because of the difficulty of manipulating identified cells. Using confocal time-lapse and cell ablation in the whole animal, we provide direct evidence for a discrete number of Drosophila peripheral glial cells acting as pioneers and guiding the rest of the migratory chain. These cells are in direct contact with several follower cells through a very long and stable cytoplasmic extension. The presence of pioneer cells and homotypic interactions at the tip of the chain allows coordinated movement and the formation of a continuous sheath around the nerve. These in vivo data open novel perspectives for understanding the cellular bases of vertebrate glial migration in physiological and pathological conditions.
Figures






References
-
- Aigouy B, Van de Bor V, Boeglin M, Giangrande A. Time-lapse and cell ablation reveal the role of cell interactions in fly glia migration and proliferation. Development. 2004;131:5127–5138. - PubMed
-
- Bevis BJ, Glick BS. Rapidly maturing variants of the Discosoma red fluorescent protein (DsRed) Nat Biotechnol. 2002;20:83–87. - PubMed
-
- Chitnis AB, Kuwada JY. Elimination of a brain tract increases errors in pathfinding by follower growth cones in the zebrafish embryo. Neuron. 1991;7:277–285. - PubMed
-
- Giangrande A. Glia in the fly wing are clonally related to epithelial cells and use the nerve as a pathway for migration. Development. 1994;120:523–534.
-
- Giangrande A, Murray MA, Palka J. Development and organization of glial cells in the peripheral nervous system of Drosophila melanogaster. Development. 1993;117:895–904. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
