Selection of a trioxaquine as an antimalarial drug candidate
- PMID: 18987321
- PMCID: PMC2579890
- DOI: 10.1073/pnas.0804338105
Selection of a trioxaquine as an antimalarial drug candidate
Abstract
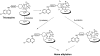
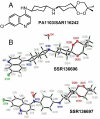
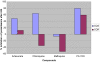
Trioxaquines are antimalarial agents based on hybrid structures with a dual mode of action. One of these molecules, PA1103/SAR116242, is highly active in vitro on several sensitive and resistant strains of Plasmodium falciparum at nanomolar concentrations (e.g., IC(50) value = 10 nM with FcM29, a chloroquine-resistant strain) and also on multidrug-resistant strains obtained from fresh patient isolates in Gabon. This molecule is very efficient by oral route with a complete cure of mice infected with chloroquine-sensitive or chloroquine-resistant strains of Plasmodia at 26-32 mg/kg. This compound is also highly effective in humanized mice infected with P. falciparum. Combined with a good drug profile (preliminary absorption, metabolism, and safety parameters), these data were favorable for the selection of this particular trioxaquine for development as drug candidate among 120 other active hybrid molecules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Greenwood BM, Bojang K, Whitty CJ, Targett GA. Malaria. Lancet. 2005;365:1487–1498. - PubMed
-
- White NJ, et al. Averting a malaria disaster. Lancet. 1999;353:1965–1967. - PubMed
-
- Nwaka S, Ridley RG. Virtual drug discovery and development for neglected diseases through public-private partnerships. Nat Rev Drug Discov. 2003;2:919–928. - PubMed
-
- Robert A, Cazelles J, Dechy-Cabaret O, Meunier B. From mechanistic studies on artemisinin derivatives to new modular antimalarial drugs. Acc Chem Res. 2002;35:167–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

