Distinct apical and basolateral membrane requirements for stretch-induced membrane traffic at the apical surface of bladder umbrella cells
- PMID: 18987341
- PMCID: PMC2613117
- DOI: 10.1091/mbc.e08-04-0439
Distinct apical and basolateral membrane requirements for stretch-induced membrane traffic at the apical surface of bladder umbrella cells
Abstract
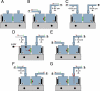
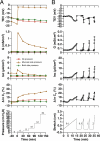
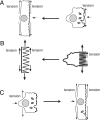
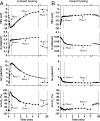
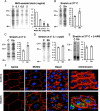
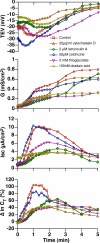
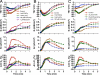
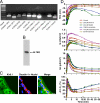
Epithelial cells respond to mechanical stimuli by increasing exocytosis, endocytosis, and ion transport, but how these processes are initiated and coordinated and the mechanotransduction pathways involved are not well understood. We observed that in response to a dynamic mechanical environment, increased apical membrane tension, but not pressure, stimulated apical membrane exocytosis and ion transport in bladder umbrella cells. The exocytic response was independent of temperature but required the cytoskeleton and the activity of a nonselective cation channel and the epithelial sodium channel. The subsequent increase in basolateral membrane tension had the opposite effect and triggered the compensatory endocytosis of added apical membrane, which was modulated by opening of basolateral K(+) channels. Our results indicate that during the dynamic processes of bladder filling and voiding apical membrane dynamics depend on sequential and coordinated mechanotransduction events at both membrane domains of the umbrella cell.
Figures

Similar articles
-
Stretch-regulated exocytosis/endocytosis in bladder umbrella cells.Mol Biol Cell. 2002 Mar;13(3):830-46. doi: 10.1091/mbc.01-09-0435. Mol Biol Cell. 2002. PMID: 11907265 Free PMC article.
-
Analysis of hydrostatic pressure-induced changes in umbrella cell surface area.Methods. 2003 Jul;30(3):207-17. doi: 10.1016/s1046-2023(03)00027-6. Methods. 2003. PMID: 12798135
-
Examining the role of mechanosensitive ion channels in pressure mechanotransduction in rat bladder urothelial cells.Ann Biomed Eng. 2011 Feb;39(2):688-97. doi: 10.1007/s10439-010-0203-3. Epub 2010 Nov 23. Ann Biomed Eng. 2011. PMID: 21104316
-
Apical plasma membrane traffic in superficial cells of bladder urothelium.Ann N Y Acad Sci. 2009 Jan;1152:18-29. doi: 10.1111/j.1749-6632.2008.04004.x. Ann N Y Acad Sci. 2009. PMID: 19161373 Review.
-
Modulation of membrane traffic by mechanical stimuli.Am J Physiol Renal Physiol. 2002 Feb;282(2):F179-90. doi: 10.1152/ajprenal.2002.282.2.F179. Am J Physiol Renal Physiol. 2002. PMID: 11788431 Review.
Cited by
-
The urothelium: a multi-faceted barrier against a harsh environment.Mucosal Immunol. 2022 Jun;15(6):1127-1142. doi: 10.1038/s41385-022-00565-0. Epub 2022 Sep 30. Mucosal Immunol. 2022. PMID: 36180582 Free PMC article. Review.
-
Recurrent Urinary Tract Infection: A Mystery in Search of Better Model Systems.Front Cell Infect Microbiol. 2021 May 26;11:691210. doi: 10.3389/fcimb.2021.691210. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34123879 Free PMC article. Review.
-
[Renal pelvic carcinoma: a different urothelial tumor?].Pathologe. 2009 Dec;30 Suppl 2:185-7. doi: 10.1007/s00292-009-1220-6. Pathologe. 2009. PMID: 19795126 Review. German.
-
Mechanotransduction in the urothelium: ATP signalling and mechanoreceptors.Heliyon. 2023 Aug 23;9(9):e19427. doi: 10.1016/j.heliyon.2023.e19427. eCollection 2023 Sep. Heliyon. 2023. PMID: 37674847 Free PMC article. Review.
-
Polarized ATP distribution in urothelial mucosal and serosal space is differentially regulated by stretch and ectonucleotidases.Am J Physiol Renal Physiol. 2015 Nov 15;309(10):F864-72. doi: 10.1152/ajprenal.00175.2015. Epub 2015 Sep 2. Am J Physiol Renal Physiol. 2015. PMID: 26336160 Free PMC article.
References
-
- Acharya P., Beckel J., Ruiz W. G., Wang E., Rojas R., Birder L., Apodaca G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am. J. Physiol. Renal Physiol. 2004;287:F305–F318. - PubMed
-
- Alenghat F. J., Nauli S. M., Kolb R., Zhou J., Ingber D. E. Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp. Cell Res. 2004;301:23–30. - PubMed
-
- Althaus M., Bogdan R., Clauss W. G., Fronius M. Mechano-sensitivity of epithelial sodium channels (ENaCs): laminar shear stress increases ion channel open probability. FASEB J. 2007;21:2389–2399. - PubMed
-
- Apodaca G. Modulation of membrane traffic by mechanical stimuli. Am. J. Physiol. Renal Physiol. 2002;282:F179–F190. - PubMed
-
- Apodaca G. The uroepithelium: not just a passive barrier. Traffic. 2004;5:117–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

