Molecular clustering of STIM1 with Orai1/CRACM1 at the plasma membrane depends dynamically on depletion of Ca2+ stores and on electrostatic interactions
- PMID: 18987344
- PMCID: PMC2613096
- DOI: 10.1091/mbc.e07-11-1132
Molecular clustering of STIM1 with Orai1/CRACM1 at the plasma membrane depends dynamically on depletion of Ca2+ stores and on electrostatic interactions
Abstract
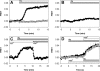
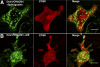
Activation of store operated Ca(2+) entry involves stromal interaction molecule 1 (STIM1), localized to the endoplasmic reticulum (ER), and calcium channel subunit (Orai1/CRACM1), localized to the plasma membrane. Confocal microscopy shows that thapsigargin-mediated depletion of ER Ca(2+) stores in RBL mast cells causes a redistribution of STIM1, labeled with monomeric red fluorescent protein (mRFP), to micrometer-scale ER-plasma membrane junctions that contain Orai1/CRACM1, labeled with monomeric Aequorea coerulescens green fluorescent protein (AcGFP). Using fluorescence resonance energy transfer (FRET), we determine that this visualized coredistribution is accompanied by nanoscale interaction of STIM1-mRFP and AcGFP-Orai1/CRACM1. We find that antigen stimulation of immunoglobulin E receptors causes much less Orai1/CRACM1 and STIM1 association, but strong interaction is observed under conditions that prevent refilling of ER stores. Stimulated association monitored by FRET is inhibited by sphingosine derivatives in parallel with inhibition of Ca(2+) influx. Similar structural and functional effects are caused by mutation of acidic residues in the cytoplasmic segment of Orai1/CRACM1, suggesting a role for electrostatic interactions via these residues in the coupling of Orai1/CRACM1 to STIM1. Our results reveal dynamic molecular interactions between STIM1 and Orai1/CRACM1 that depend quantitatively on electrostatic interactions and on the extent of store depletion.
Figures



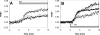

References
-
- Baba Y., Nishida K., Fujii Y., Hirano T., Hikida M., Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat. Immunol. 2008;9:81–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

