Distinctive localization of antigen-presenting cells in human lymph nodes
- PMID: 18987360
- PMCID: PMC2687552
- DOI: 10.1182/blood-2008-06-165266
Distinctive localization of antigen-presenting cells in human lymph nodes
Abstract
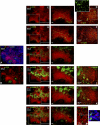
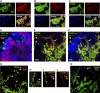
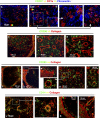
Professional antigen-presenting cells (APCs) are sentinel cells of the immune system that present antigen to T lymphocytes and mediate an appropriate immune response. It is therefore surprising that knowledge of the professional APCs in human lymph nodes is limited. Using 3-color immunohistochemistry, we have identified APCs in human lymph nodes, excluding plasmacytoid APCs, that fall into 2 nonoverlapping classes: (1) CD209+ APCs, coexpressing combinations of CD206, CD14, and CD68, that occupied the medullary cords, lined the capsule and trabeculae and were also scattered throughout the diffuse T-lymphocyte areas of the paracortex; and (2) APCs expressing combinations of CD1a, CD207, and CD208, that were always restricted to the paracortex. Surprisingly, this second class of APCs was almost entirely absent from many lymph nodes. Our data suggest that most CD208+ cells, often referred to as "interdigitating cells," derive from migratory APCs, and that the major APC subset consistently resident in the paracortex of human lymph nodes is the CD209+ subset. All APC subsets were demonstrated to be in close contact with the fibroreticular network. The identification of 2 distinct APC populations in the paracortex of human lymph nodes has important implications for understanding T-lymphocyte responses and optimizing vaccine design.
Figures



References
-
- Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. - PubMed
-
- Allan RS, Waithman J, Bedoui S, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. - PubMed
-
- Henri S, Vremec D, Kamath A, et al. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–748. - PubMed
-
- Kissenpfennig A, Henri S, Dubois B, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. - PubMed
-
- Allan RS, Smith CM, Belz GT, et al. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

