Thiopurine methyltransferase activity is related to the risk of relapse of childhood acute lymphoblastic leukemia: results from the NOPHO ALL-92 study
- PMID: 18987654
- PMCID: PMC3898327
- DOI: 10.1038/leu.2008.316
Thiopurine methyltransferase activity is related to the risk of relapse of childhood acute lymphoblastic leukemia: results from the NOPHO ALL-92 study
Abstract
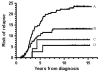
Myelotoxicity during thiopurine therapy is enhanced in patients, who because of single nucleotide polymorphisms have decreased activity of the enzyme thiopurine methyltransferase (TPMT) and thus more thiopurine converted into 6-thioguanine nucleotides. Of 601 children with acute lymphoblastic leukemia (ALL) who were treated by the NOPHO ALL-92 protocol, 117 had TPMT genotype determined, whereas for 484 patients only erythrocyte TPMT activity was available. The latter were classified as heterozygous, if TPMT activity was <14 IU/ml, or deficient (<1.0 IU/ml). 526 patients had TPMT wild type, 73 were presumed heterozygous, and two were TPMT deficient. Risk of relapse was higher for the 526 TPMT wild type patients than for the remaining 75 patients (18 vs 7%, P=0.03). In Cox multivariate regression analysis, sex (male worse; P=0.06), age (higher age worse, P=0.02), and TPMT activity (wild type worse; P=0.02) were related to risk of relapse. Despite a lower probability of relapse, patients in the low TPMT activity group did not have superior survival (P=0.82), possibly because of an excess of secondary cancers among these 75 patients (P=0.07). These data suggest that children with ALL and TPMT wild type might have their cure rate improved, if the pharmacokinetics/-dynamics of TPMT low-activity patients could be mimicked without a concurrent excessive risk of second cancers.
Figures

 =oral prednisolone (60 mg/m2/day). v & V=vincristine (2.0 mg/m2). A=doxorubicin (40 mg/m2 i.v.),
=oral prednisolone (60 mg/m2/day). v & V=vincristine (2.0 mg/m2). A=doxorubicin (40 mg/m2 i.v.),  =Erwinia asparaginase (30 000 IU/m2 per day for 10 days). o=intrathecal MTX by age. M=high-dose MTX (5 g/m2/24 h or 8 g/m2 (HR only)). V/P=vincristine (2.0 mg/m2 × 1)/prednisolone (60 mg/m2/day for 7 days) reinductions.
=Erwinia asparaginase (30 000 IU/m2 per day for 10 days). o=intrathecal MTX by age. M=high-dose MTX (5 g/m2/24 h or 8 g/m2 (HR only)). V/P=vincristine (2.0 mg/m2 × 1)/prednisolone (60 mg/m2/day for 7 days) reinductions.  MTX 20 mg/m2/week.
MTX 20 mg/m2/week.  6MP 75 mg/m2/day. c=cyclophosphamide (1 g/m2 i.v.). Low-dose AraC (75 mg/m2).
6MP 75 mg/m2/day. c=cyclophosphamide (1 g/m2 i.v.). Low-dose AraC (75 mg/m2).  6MP or 6TG 60 mg/m2/day. U=daunorubicin (30 mg/m2),
6MP or 6TG 60 mg/m2/day. U=daunorubicin (30 mg/m2),  =Erwinia asparaginase (30.000 IU/m2 two times weekly for 2 weeks). HC=high dose AraC (2 g/m2 two times daily for 3 days). v/p=vincristine (2.0 mg/m2 × 1)/prednisolone (40 mg/m2/day for 7 days) reinductions without or with (/m) methotrexate i.t. in age-adjusted doses.
=Erwinia asparaginase (30.000 IU/m2 two times weekly for 2 weeks). HC=high dose AraC (2 g/m2 two times daily for 3 days). v/p=vincristine (2.0 mg/m2 × 1)/prednisolone (40 mg/m2/day for 7 days) reinductions without or with (/m) methotrexate i.t. in age-adjusted doses.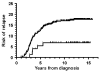
Similar articles
-
Pharmacogenetically based dosing of thiopurines in childhood acute lymphoblastic leukemia: influence on cure rates and risk of second cancer.Pediatr Blood Cancer. 2014 May;61(5):797-802. doi: 10.1002/pbc.24921. Epub 2014 Jan 3. Pediatr Blood Cancer. 2014. PMID: 24395436
-
Pharmacokinetics of 6-Thioguanine and 6-Mercaptopurine Combination Maintenance Therapy of Childhood ALL: Hypothesis and Case Report.J Pediatr Hematol Oncol. 2015 Apr;37(3):e206-9. doi: 10.1097/MPH.0000000000000246. J Pediatr Hematol Oncol. 2015. PMID: 25171455
-
Genetic polymorphism of thiopurine methyltransferase and its clinical relevance for childhood acute lymphoblastic leukemia.Leukemia. 2000 Apr;14(4):567-72. doi: 10.1038/sj.leu.2401723. Leukemia. 2000. PMID: 10764140 Review.
-
Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus.J Natl Cancer Inst. 1999 Dec 1;91(23):2001-8. doi: 10.1093/jnci/91.23.2001. J Natl Cancer Inst. 1999. PMID: 10580024 Clinical Trial.
-
[Therapeutic drug monitoring of 6-thioguanine nucleotides in paediatric acute lymphoblastic leukaemia: interest and limits].Therapie. 2010 May-Jun;65(3):187-93. doi: 10.2515/therapie/2010031. Epub 2010 Aug 11. Therapie. 2010. PMID: 20699069 Review. French.
Cited by
-
No association between relapse hazard and thiopurine methyltransferase geno- or phenotypes in non-high risk acute lymphoblastic leukemia: a NOPHO ALL2008 sub-study.Cancer Chemother Pharmacol. 2021 Aug;88(2):271-279. doi: 10.1007/s00280-021-04281-7. Epub 2021 Apr 29. Cancer Chemother Pharmacol. 2021. PMID: 33928426
-
Challenges in implementing individualized medicine illustrated by antimetabolite therapy of childhood acute lymphoblastic leukemia.Clin Proteomics. 2011 Jun 3;8(1):8. doi: 10.1186/1559-0275-8-8. Clin Proteomics. 2011. PMID: 21906358 Free PMC article.
-
Optimal chemotherapy for leukemia: a model-based strategy for individualized treatment.PLoS One. 2014 Oct 13;9(10):e109623. doi: 10.1371/journal.pone.0109623. eCollection 2014. PLoS One. 2014. PMID: 25310465 Free PMC article.
-
Bayesian hierarchical lasso Cox model: A 9-gene prognostic signature for overall survival in gastric cancer in an Asian population.PLoS One. 2022 Apr 14;17(4):e0266805. doi: 10.1371/journal.pone.0266805. eCollection 2022. PLoS One. 2022. PMID: 35421138 Free PMC article.
-
The association of reduced folate carrier 80G>A polymorphism to outcome in childhood acute lymphoblastic leukemia interacts with chromosome 21 copy number.Blood. 2010 Jun 10;115(23):4671-7. doi: 10.1182/blood-2010-01-256958. Epub 2010 Mar 24. Blood. 2010. PMID: 20335220 Free PMC article. Clinical Trial.
References
-
- Davidsen ML, Dalhoff K, Schmiegelow K. Pharmacogenetics influence treatment efficacy in childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2008 (in press) - PubMed
-
- Burchenal JH, Murphy ML, Ellison RR, Sykes MP, Tan TC, Leone LA, et al. Clinical evaluation of a new antimetabolite, 6-mercaptopurine, in the treatment of leukemia and allied diseases. Blood. 1953;8:965–987. - PubMed
-
- Schmiegelow K, Gustafsson G. Acute lymphoblastic leukemia. In: Voute PA, Barrett A, Stevens MCG, Caron M, editors. Cancer in Children. 5th edn Oxford University Press; Oxford: 2005. pp. 138–170.
-
- Coulthard S, Hogarth L. The thiopurines: an update. Invest New Drugs. 2005;23:523–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

