Sustained release of antibiotics from injectable and thermally responsive polypeptide depots
- PMID: 18988275
- PMCID: PMC2694231
- DOI: 10.1002/jbm.b.31254
Sustained release of antibiotics from injectable and thermally responsive polypeptide depots
Abstract
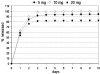
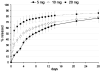
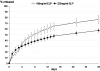
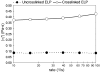
Biodegradable polymeric scaffolds are of interest for delivering antibiotics to local sites of infection in orthopaedic applications, such as bone and diarthrodial joints. The objective of this study was to develop a biodegradable scaffold with ease of drug loading in aqueous solution, while providing for drug depot delivery via syringe injection. Elastin-like polypeptides (ELPs) were used for this application, biopolymers of repeating pentapeptide sequences that were thermally triggered to undergo in situ depot formation at body temperature. ELPs were modified to enable loading with the antibiotics, cefazolin, and vancomycin, followed by induction of the phase transition in vitro. Cefazolin and vancomycin concentrations were monitored, as well as bioactivity of the released antibiotics, to test an ability of the ELP depot to provide for prolonged release of bioactive drugs. Further tests of formulation viscosity were conducted to test suitability as an injectable drug carrier. Results demonstrate sustained release of therapeutic concentrations of bioactive antibiotics by the ELP, with first-order time constants for drug release of approximately 25 h for cefazolin and approximately 500 h for vancomycin. These findings illustrate that an injectable, in situ forming ELP depot can provide for sustained release of antibiotics with an effect that varies across antibiotic formulation. ELPs have important advantages for drug delivery, as they are known to be biocompatible, biodegradable, and elicit no known immune response. These benefits suggest distinct advantages over currently used carriers for antibiotic drug delivery in orthopedic applications.
(c) 2008 Wiley Periodicals, Inc.
Figures
References
-
- Darouiche RO. Current concepts - Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–1429. - PubMed
-
- Buchholz HW, Elson RA, Engelbrecht E, Lodenkamper H, Rottger J, Siegel A. Management of Deep Infection of Total Hip-Replacement. J Bone Joint Surg (Br) Volume. 1981;63(3):342–353. - PubMed
-
- Heijink A, Yaszemski MJ, Patel R, Rouse MS, Lewallen DG, Hanssen AD. Local antibiotic delivery with OsteoSet (R), DBX (R), and Collagraft (R) Clin Orthop. 2006;451:29–33. - PubMed
-
- Garvin KL, Miyano JA, Robinson D, Giger D, Novak J, Radio S. Polylactide/polyglycolide antibiotic implants in the treatment of osteomyelitis. A canine model. J Bone Joint Surg (Am) 1994;76(10):1500–6. - PubMed
-
- Garvin K, Feschuk C. Polylactide-polyglycolide antibiotic implants. Clin Orthop. 2005;437:105–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

