S-glutathionylation impairs signal transducer and activator of transcription 3 activation and signaling
- PMID: 18988672
- PMCID: PMC2654735
- DOI: 10.1210/en.2008-1241
S-glutathionylation impairs signal transducer and activator of transcription 3 activation and signaling
Abstract
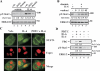
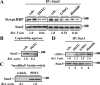
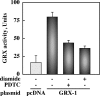
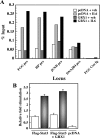
S-glutathionylation is a physiological, reversible protein modification of cysteine residues with glutathione in response to mild oxidative stress. Because the key cell growth regulator signal transducer and activator of transcription (STAT) 3 is particularly susceptible to redox regulation, we hypothesized that oxidative modification of cysteine residues of STAT3 by S-glutathionylation may occur. Herein, we show that the cysteine residues of STAT3 are modified by a thiol-alkylating agent and are the targets of S-glutathionylation. STAT3 protein thiol reactivity was reversibly attenuated with concomitant increase in the S-glutathionylation of STAT3 upon treatment of human HepG2 hepatoma cells with pyrrolidine dithiocarbamate, glutathione disulfide, or diamide. Under these conditions there was a marked reduction in IL-6-dependent STAT3 signaling, including decreased STAT3 tyrosine phosphorylation, loss in nuclear accumulation of STAT3, and impaired expression of target genes, such as fibrinogen-gamma. In a cell-free system, diamide induced glutathionylation of STAT3, which was decreased upon addition of glutaredoxin (GRX)-1, a deglutathionylation enzyme, or the reducing agent, dithiothreitol. Glutathionylated STAT3 was a poor Janus protein tyrosine kinase 2 substrate in vitro, and it exhibited low DNA-binding activity. Cellular GRX-1 activity was inhibited by diamide and pyrrolidine dithiocarbamate treatment; however, ectopic expression of GRX-1 was accompanied by a modest increase in phosphorylation, nuclear translocation, and DNA-binding ability of STAT3 in response to IL-6. These results are the first to show S-glutathionylation of STAT3, a modification that may exert regulatory function in STAT3 signaling.
Figures


Similar articles
-
S-Glutathionylation at Cys328 and Cys542 impairs STAT3 phosphorylation.ACS Chem Biol. 2014 Aug 15;9(8):1885-93. doi: 10.1021/cb500407d. Epub 2014 Jun 27. ACS Chem Biol. 2014. PMID: 24941337
-
S-glutathionylation exerts opposing roles in the regulation of STAT1 and STAT3 signaling in reactive microglia.Free Radic Biol Med. 2018 Mar;117:191-201. doi: 10.1016/j.freeradbiomed.2018.02.005. Epub 2018 Feb 8. Free Radic Biol Med. 2018. PMID: 29427792
-
Redox modulation of endothelial nitric oxide synthase by glutaredoxin-1 through reversible oxidative post-translational modification.Biochemistry. 2013 Sep 24;52(38):6712-23. doi: 10.1021/bi400404s. Epub 2013 Sep 11. Biochemistry. 2013. PMID: 23977830 Free PMC article.
-
Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress.Curr Opin Pharmacol. 2007 Aug;7(4):381-91. doi: 10.1016/j.coph.2007.06.003. Epub 2007 Jul 26. Curr Opin Pharmacol. 2007. PMID: 17662654 Review.
-
Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation.Antioxid Redox Signal. 2005 Mar-Apr;7(3-4):348-66. doi: 10.1089/ars.2005.7.348. Antioxid Redox Signal. 2005. PMID: 15706083 Review.
Cited by
-
Depletion of cellular glutathione modulates LIF-induced JAK1-STAT3 signaling in cardiac myocytes.Int J Biochem Cell Biol. 2012 Dec;44(12):2106-15. doi: 10.1016/j.biocel.2012.08.016. Epub 2012 Aug 22. Int J Biochem Cell Biol. 2012. PMID: 22939972 Free PMC article.
-
Cysteine Glutathionylation Acts as a Redox Switch in Endothelial Cells.Antioxidants (Basel). 2019 Aug 16;8(8):315. doi: 10.3390/antiox8080315. Antioxidants (Basel). 2019. PMID: 31426416 Free PMC article. Review.
-
Development of 'Redox Arrays' for identifying novel glutathionylated proteins in the secretome.Sci Rep. 2015 Sep 29;5:14630. doi: 10.1038/srep14630. Sci Rep. 2015. PMID: 26416726 Free PMC article.
-
Hemistepsin a Induces Apoptosis of Hepatocellular Carcinoma Cells by Downregulating STAT3.Int J Mol Sci. 2021 Apr 29;22(9):4743. doi: 10.3390/ijms22094743. Int J Mol Sci. 2021. PMID: 33947048 Free PMC article.
-
STAT proteins: a kaleidoscope of canonical and non-canonical functions in immunity and cancer.J Hematol Oncol. 2021 Nov 22;14(1):198. doi: 10.1186/s13045-021-01214-y. J Hematol Oncol. 2021. PMID: 34809691 Free PMC article. Review.
References
-
- Kishimoto T 2005 Interleukin-6: from basic science to medicine—40 years in immunology. Annu Rev Immunol 23:1–21 - PubMed
-
- Schuringa JJ, Schepers H, Vellenga E, Kruijer W 2001 Ser727-dependent transcriptional activation by association of p300 with STAT3 upon IL-6 stimulation. FEBS Lett 495:71–76 - PubMed
-
- Kortylewski M, Feld F, Krüger KD, Bahrenberg G, Roth RA, Joost HG, Heinrich PC, Behrmann I, Barthel A 2003 Akt modulates STAT3-mediated gene expression through a FKHR (FOXO1a)-dependent mechanism. J Biol Chem 278:5242–5249 - PubMed
-
- Lufei C, Koh TH, Uchida T, Cao X 2007 Pin1 is required for the Ser727 phosphorylation-dependent Stat3 activity. Oncogene 26:7656–7664 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

