Gliotypic neural stem cells transiently adopt tumorigenic properties during normal differentiation
- PMID: 18988710
- PMCID: PMC4425277
- DOI: 10.1634/stemcells.2008-0842
Gliotypic neural stem cells transiently adopt tumorigenic properties during normal differentiation
Abstract
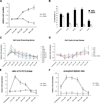
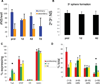
An increasing body of evidence suggests that astrocytic gliomas of the central nervous system may be derived from gliotypic neural stem cells. To date, the study of these tumors, particularly the identification of originating cellular population(s), has been frustrated by technical difficulties in accessing the native niche of stem cells. To identify any hallmark signs of cancer in neural stem cells or their progeny, we cultured subventricular zone-derived tissue in a unique in vitro model that temporally and phenotypically recapitulates adult neurogenesis. Contrary to some reports, we found undifferentiated neural stem cells possess few characteristics, suggesting prototumorigenic potential. However, when induced to differentiate, neural stem cells give rise to intermediate progenitors that transiently exhibit multiple glioma characteristics, including aneuploidy, loss of growth-contact inhibition, alterations in cell cycle, and growth factor insensitivity. Further examination of progenitor populations revealed a subset of cells defined by the aberrant expression of (the pathological glioma marker) class III beta-tubulin that exhibit intrinsic parental properties of gliomas, including multilineage differentiation and continued proliferation in the absence of a complex cellular regulatory environment. As tumorigenic characteristics in progenitor cells normally disappear with the generation of mature progeny, this suggests that developmentally intermediate progenitor cells, rather than neural stem cells, may be the origin of so-called "stem cell-derived" tumors.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures


Similar articles
-
Fetal human hematopoietic stem cells can differentiate sequentially into neural stem cells and then astrocytes in vitro.J Hematother Stem Cell Res. 2003 Feb;12(1):23-32. doi: 10.1089/152581603321210109. J Hematother Stem Cell Res. 2003. PMID: 12662433
-
Sphere formation of ocular epithelial cells in the ciliary body is a reprogramming system for neural differentiation.Brain Res. 2006 Jun 6;1093(1):54-70. doi: 10.1016/j.brainres.2006.03.093. Epub 2006 May 11. Brain Res. 2006. PMID: 16697356
-
Transforming growth factor alpha promotes sequential conversion of mature astrocytes into neural progenitors and stem cells.Oncogene. 2007 Apr 26;26(19):2695-706. doi: 10.1038/sj.onc.1210071. Epub 2006 Oct 23. Oncogene. 2007. PMID: 17057735
-
Class III beta-tubulin isotype: a key cytoskeletal protein at the crossroads of developmental neurobiology and tumor neuropathology.J Child Neurol. 2003 Dec;18(12):851-66; discussion 867. doi: 10.1177/088307380301801205. J Child Neurol. 2003. PMID: 14736079 Review.
-
Class III beta-tubulin in human development and cancer.Cell Motil Cytoskeleton. 2003 Jun;55(2):77-96. doi: 10.1002/cm.10116. Cell Motil Cytoskeleton. 2003. PMID: 12740870 Review.
Cited by
-
Role and mechanism of neural stem cells of the subventricular zone in glioblastoma.World J Stem Cells. 2021 Jul 26;13(7):877-893. doi: 10.4252/wjsc.v13.i7.877. World J Stem Cells. 2021. PMID: 34367482 Free PMC article. Review.
-
Derivation of neural stem cells from an animal model of psychiatric disease.Transl Psychiatry. 2013 Nov 5;3(11):e323. doi: 10.1038/tp.2013.96. Transl Psychiatry. 2013. PMID: 24193728 Free PMC article.
-
A new role for interferon gamma in neural stem/precursor cell dysregulation.Mol Neurodegener. 2011 Mar 3;6:18. doi: 10.1186/1750-1326-6-18. Mol Neurodegener. 2011. PMID: 21371330 Free PMC article.
-
The Ets protein Pointed prevents both premature differentiation and dedifferentiation of Drosophila intermediate neural progenitors.Development. 2016 Sep 1;143(17):3109-18. doi: 10.1242/dev.137281. Epub 2016 Aug 10. Development. 2016. PMID: 27510969 Free PMC article.
-
Astrocytes reverted to a neural progenitor-like state with transforming growth factor alpha are sensitized to cancerous transformation.Stem Cells. 2009 Oct;27(10):2373-82. doi: 10.1002/stem.155. Stem Cells. 2009. PMID: 19544474 Free PMC article.
References
-
- Kukekov VG, Laywell ED, Suslov O, et al. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333–344. - PubMed
-
- Ignatova TN, Kukekov VG, Laywell ED, et al. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. - PubMed
-
- Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. - PubMed
-
- Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

