Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues
- PMID: 18988807
- PMCID: PMC2626399
- DOI: 10.2353/ajpath.2008.080378
Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues
Abstract
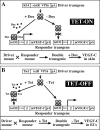
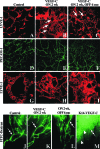
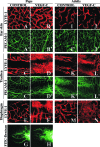
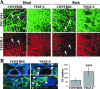
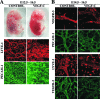
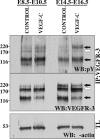
Vascular endothelial growth factor-C (VEGF-C) is the quintessential lymphangiogenic growth factor that is required for the development of the lymphatic system and is capable of stimulating lymphangiogenesis in adults by activating its receptor, VEGFR-3. Although VEGF-C is a major candidate molecule for the development of prolymphangiogenic therapy for defective lymphatic vessels in lymphedema, the stability of lymph vessels generated by exogenous VEGF-C administration is not currently known. We studied VEGF-C-stimulated lymphangiogenesis in inducible transgenic mouse models in which growth factor expression can be spatially and temporally controlled without side effects, such as inflammation. VEGF-C induction in adult mouse skin for 1 to 2 weeks caused robust lymphatic hyperplasia that persisted for at least 6 months. VEGF-C induced lymphangiogenesis in numerous tissues and organs when expressed in the vascular endothelium in either neonates or adult mice. Very few or no effects were observed in either blood vessels or collecting lymph vessels. Additionally, VEGF-C stimulated lymphangiogenesis in embryos after the onset of lymphatic vessel development. Strikingly, a strong angiogenic effect was observed after VEGF-C induction in vascular endothelium at any point before embryonic day 16.5. Our results indicate that blood vessels can undergo VEGF-C-induced angiogenesis even after down-regulation of VEGFR-3 in embryos; however, transient VEGF-C expression in adults can induce long-lasting lymphatic hyperplasia with no obvious side effects on the blood vasculature.
Figures
Similar articles
-
The tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesis.Cancer Res. 2008 Jun 15;68(12):4754-62. doi: 10.1158/0008-5472.CAN-07-5809. Cancer Res. 2008. PMID: 18559522
-
Molecular control of lymphatic metastasis.Ann N Y Acad Sci. 2008;1131:225-34. doi: 10.1196/annals.1413.020. Ann N Y Acad Sci. 2008. PMID: 18519975 Review.
-
Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice.EMBO J. 2001 Mar 15;20(6):1223-31. doi: 10.1093/emboj/20.6.1223. EMBO J. 2001. PMID: 11250889 Free PMC article.
-
Expression of VEGFR-3 and 5'-nase in regenerating lymphatic vessels of the cutaneous wound healing.Microsc Res Tech. 2004 Jun 15;64(3):279-86. doi: 10.1002/jemt.20082. Microsc Res Tech. 2004. PMID: 15452895
-
Lymphangiogenic growth factors, receptors and therapies.Thromb Haemost. 2003 Aug;90(2):167-84. doi: 10.1160/TH03-04-0200. Thromb Haemost. 2003. PMID: 12888864 Review.
Cited by
-
Rapamycin reversal of VEGF-C-driven lymphatic anomalies in the respiratory tract.JCI Insight. 2017 Aug 17;2(16):e90103. doi: 10.1172/jci.insight.90103. eCollection 2017 Aug 17. JCI Insight. 2017. PMID: 28814666 Free PMC article.
-
Afferent Lymphatic Transport and Peripheral Tissue Immunity.J Immunol. 2021 Jan 15;206(2):264-272. doi: 10.4049/jimmunol.2001060. J Immunol. 2021. PMID: 33397740 Free PMC article. Review.
-
Structural determinants of growth factor binding and specificity by VEGF receptor 2.Proc Natl Acad Sci U S A. 2010 Feb 9;107(6):2425-30. doi: 10.1073/pnas.0914318107. Proc Natl Acad Sci U S A. 2010. PMID: 20145116 Free PMC article.
-
Lymphatic-specific intracellular modulation of receptor tyrosine kinase signaling improves lymphatic growth and function.Sci Signal. 2021 Aug 10;14(695):eabc0836. doi: 10.1126/scisignal.abc0836. Sci Signal. 2021. PMID: 34376570 Free PMC article.
-
Vascular endothelial growth factor C attenuates joint damage in chronic inflammatory arthritis by accelerating local lymphatic drainage in mice.Arthritis Rheum. 2011 Aug;63(8):2318-28. doi: 10.1002/art.30421. Arthritis Rheum. 2011. PMID: 21538325 Free PMC article.
References
-
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Ebenhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. - PubMed
-
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. - PubMed
-
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. - PubMed
-
- Oh SJ, Jeltsch MM, Birkenhager R, McCarthy JE, Weich HA, Christ B, Alitalo K, Wilting J. VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev Biol. 1997;188:96–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

