Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway
- PMID: 18988856
- PMCID: PMC2652400
- DOI: 10.1126/science.1161566
Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway
Abstract
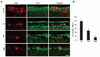
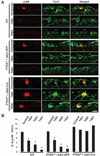
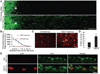
The failure of axons to regenerate is a major obstacle for functional recovery after central nervous system (CNS) injury. Removing extracellular inhibitory molecules results in limited axon regeneration in vivo. To test for the role of intrinsic impediments to axon regrowth, we analyzed cell growth control genes using a virus-assisted in vivo conditional knockout approach. Deletion of PTEN (phosphatase and tensin homolog), a negative regulator of the mammalian target of rapamycin (mTOR) pathway, in adult retinal ganglion cells (RGCs) promotes robust axon regeneration after optic nerve injury. In wild-type adult mice, the mTOR activity was suppressed and new protein synthesis was impaired in axotomized RGCs, which may contribute to the regeneration failure. Reactivating this pathway by conditional knockout of tuberous sclerosis complex 1, another negative regulator of the mTOR pathway, also leads to axon regeneration. Thus, our results suggest the manipulation of intrinsic growth control pathways as a therapeutic approach to promote axon regeneration after CNS injury.
Figures

Comment in
-
Neuroscience. Overcoming inhibitions.Science. 2008 Nov 7;322(5903):869-72. doi: 10.1126/science.1166152. Science. 2008. PMID: 18988832 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

