A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity
- PMID: 18988862
- PMCID: PMC2670794
- DOI: 10.1182/blood-2008-08-173195
A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity
Abstract
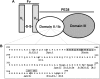
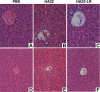
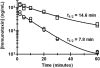
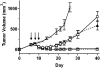
Immunotoxins based on Pseudomonas exotoxin A (PE) are promising anticancer agents that combine a variable fragment (Fv) from an antibody to a tumor-associated antigen with a 38-kDa fragment of PE (PE38). The intoxication pathway of PE immunotoxins involves receptor-mediated internalization and trafficking through endosomes/lysosomes, during which the immunotoxin undergoes important proteolytic processing steps but must otherwise remain intact for eventual transport to the cytosol. We have investigated the proteolytic susceptibility of PE38 immunotoxins to lysosomal proteases and found that cleavage clusters within a limited segment of PE38. We subsequently generated mutants containing deletions in this region using HA22, an anti-CD22 Fv-PE38 immunotoxin currently undergoing clinical trials for B-cell malignancies. One mutant, HA22-LR, lacks all identified cleavage sites, is resistant to lysosomal degradation, and retains excellent biologic activity. HA22-LR killed chronic lymphocytic leukemia cells more potently and uniformly than HA22, suggesting that lysosomal protease digestion may limit immunotoxin efficacy unless the susceptible domain is eliminated. Remarkably, mice tolerated doses of HA22-LR at least 10-fold higher than lethal doses of HA22, and these higher doses exhibited markedly enhanced antitumor activity. We conclude that HA22-LR advances the therapeutic efficacy of HA22 by using an approach that may be applicable to other PE-based immunotoxins.
Figures



References
-
- Zhang Q, Chen G, Liu X, et al. Monoclonal antibodies as therapeutic agents in oncology and antibody gene therapy. Cell Res. 2007;17:89–99. - PubMed
-
- Pastan I, Hassan R, Fitzgerald DJ, et al. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. - PubMed
-
- Pastan I, Hassan R, FitzGerald DJ, et al. Immunotoxin treatment of cancer. Annu Rev Med. 2007;58:221–237. - PubMed
-
- Kreitman RJ, Squires DR, Stetler-Stevenson M, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol. 2005;23:6719–6729. - PubMed
-
- Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I. V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

