Cardiogenic differentiation and transdifferentiation of progenitor cells
- PMID: 18988903
- PMCID: PMC2748983
- DOI: 10.1161/CIRCRESAHA.108.180588
Cardiogenic differentiation and transdifferentiation of progenitor cells
Abstract
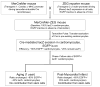
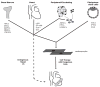
In recent years, cell transplantation has drawn tremendous interest as a novel approach to preserving or even restoring contractile function to infarcted hearts. A typical human infarct involves the loss of approximately 1 billion cardiomyocytes, and, therefore, many investigators have sought to identify endogenous or exogenous stem cells with the capacity to differentiate into committed cardiomyocytes and repopulate lost myocardium. As a result of these efforts, dozens of stem cell types have been reported to have cardiac potential. These include pluripotent embryonic stem cells, as well various adult stem cells resident in compartments including bone marrow, peripheral tissues, and the heart itself. Some of these cardiogenic progenitors have been reported to contribute replacement muscle through endogenous reparative processes or via cell transplantation in preclinical cardiac injury models. However, considerable disagreement exists regarding the efficiency and even the reality of cardiac differentiation by many of these stem cell types, making these issues a continuing source of controversy in the field. In this review, we consider approaches to cell fate mapping and establishing the cardiac phenotype, as well as the present state of the evidence for the cardiogenic and regenerative potential of the major candidate stem cell types.
Figures
References
-
- Leor J, Patterson M, Quinones MJ, Kedes LH, Kloner RA. Transplantation of fetal myocardial tissue into the infarcted myocardium of rat. A potential method for repair of infarcted myocardium? Circulation. 1996;94:II332–336. - PubMed
-
- Li RK, Jia ZQ, Weisel RD, Mickle DA, Zhang J, Mohabeer MK, Rao V, Ivanov J. Cardiomyocyte transplantation improves heart function. Ann Thorac Surg. 1996;62:654–660. discussion 660–651. - PubMed
-
- Scorsin M, Hagege AA, Marotte F, Mirochnik N, Copin H, Barnoux M, Sabri A, Samuel JL, Rappaport L, Menasche P. Does transplantation of cardiomyocytes improve function of infarcted myocardium? Circulation. 1997;96:II-188–193. - PubMed
-
- Rubart M, Pasumarthi KB, Nakajima H, Soonpaa MH, Nakajima HO, Field LJ. Physiological coupling of donor and host cardiomyocytes after cellular transplantation. Circ Res. 2003;92:1217–1224. - PubMed
-
- Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

