An auxin transport-based model of root branching in Arabidopsis thaliana
- PMID: 18989371
- PMCID: PMC2577305
- DOI: 10.1371/journal.pone.0003673
An auxin transport-based model of root branching in Arabidopsis thaliana
Abstract
Root architecture is a crucial part of plant adaptation to soil heterogeneity and is mainly controlled by root branching. The process of root system development can be divided into two successive steps: lateral root initiation and lateral root development/emergence which are controlled by different fluxes of the plant hormone auxin. While shoot architecture appears to be highly regular, following rules such as the phyllotactical spiral, root architecture appears more chaotic. We used stochastic modeling to extract hidden rules regulating root branching in Arabidopsis thaliana. These rules were used to build an integrative mechanistic model of root ramification based on auxin. This model was experimentally tested using plants with modified rhythm of lateral root initiation or mutants perturbed in auxin transport. Our analysis revealed that lateral root initiation and lateral root development/emergence are interacting with each other to create a global balance between the respective ratio of initiation and emergence. A mechanistic model based on auxin fluxes successfully predicted this property and the phenotype alteration of auxin transport mutants or plants with modified rhythms of lateral root initiation. This suggests that root branching is controlled by mechanisms of lateral inhibition due to a competition between initiation and development/emergence for auxin.
Conflict of interest statement
Figures





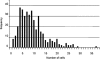
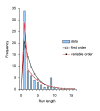
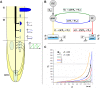


References
-
- Douady S, Couder Y. Phyllotaxis as a physical self-organized growth process. Phys Rev Lett. 1992;68:2098–2101. - PubMed
-
- Malamy JE. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell & Env. 2005;28:67–77. - PubMed
-
- Hodge A. Plastic plants and patchy soils. J Exp Bot. 2006;57:401–411. - PubMed
-
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, et al. Dissecting Arabidopsis lateral root development. Trends Plant Sci. 2003;8:165–171. - PubMed
-
- De Smet I, Vanneste S, Inzé D, Beeckman T. Lateral root initiation or the birth of a new meristem. Plant Mol Biol. 2006;60:871–887. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

